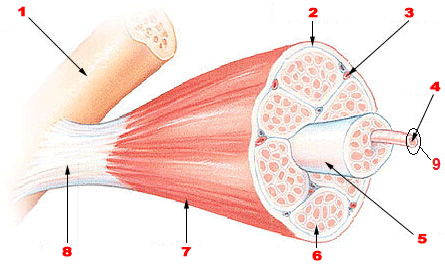
Figure 1. Skeletal Muscle Organization. Image thoroughly described below.
Gross and Microscopic Anatomy of Skeletal Muscle
Anatomy & Physiology I
Each skeletal muscle is considered an organ, and like any other organ in the body, skeletal muscles are compilations of various tissues. If you remember, there are 4 major tissue types (muscular, connective, nervous, and epithelial tissues) and you find all 4 in a skeletal muscle. This first is obvious - muscular tissue composes the bulk of this organ. The second - connective tissue - is less obvious. Each skeletal muscle has 3 layers of connective tissue called "mysia" that surround various components of the skeletal muscle and compartmentalize the muscle. We will see all 3 of these during the next segment about skeletal muscle organization. The third type of tissue is nervous tissue. By now we know that skeletal muscle is controlled voluntarily which means there must be neurons closely associated with the organ (innervation) to stimulate contraction and production of force when the intent to do so arises. These neurons are called somatic motor neurons ... soma = body and motor = producing movement, so these neurons produce movement/locomotion of the body. The last tissue type is epithelial tissue, and this one may have you scratching your heads. We know that when we move our muscles it takes energy to create that movement; therefore, each skeletal muscle cell must be capable of creating energy. Each cell contains numerous mitochondria, the organelles responsible for creating energy in the form of ATP from oxygen and glucose, but the cell also needs a blood supply that can bring those nutrients to the cell. These blood vessels are lined with simple squamous epithelium... and there is your fourth and final major tissue type.
Now that we have examined all the major tissues found in this complex organ we must now learn about how the organ is organized. Like the rest of the structures of your body, skeletal muscles are not simply thrown together, they are highly structured. Why is this organization so important? Two reasons, the organization of the intracellular proteins allows for the greatest contraction possible and the compartmentalization allows for activation of only the skeletal muscle cells needed for a particular force production. You wouldn't use the same force to lift a coffee mug as you would a couch. The compartments allow us to activate only a few muscle cells to lift the cup and then activate many more to lift the couch.
When we start to examine the structure of a whole skeletal muscle it is important to deconstruct the organ one layer at a time and after it is fully deconstructed, piece it back together from the smallest pieces into the whole organ. If you were to look at a whole skeletal muscle, like the biceps brachii, you would see that it is covered by a layer of connective tissue called the epimysium. This epimysium not only covers the entire skeletal muscle keeping its integrity as it contracts and extends, but also separates the skeletal muscle from other surrounding skeletal muscles allowing it to move independently. The epimysium also extends out and connects with the periosteum of the skeletal system to form tendons. This tendon ensures that when the skeletal muscle contracts it will pull on the attached bone and cause movement.
Next we cut the skeletal muscle along a transverse plane to see the inside of the organ. What we notice are many large bundles inside the skeletal muscle. These large bundles are called fascicles and are covered with a layer of connective tissue called the perimysium. The perimysium allows the nervous system to selectively activate muscle cells based on the need for a particular amount of force – remember the coffee mug and couch example?
If we look closely at each fascicle we will see many bundles inside of this structure that we call skeletal muscle cells or muscle fibers. Remember, both terms are widely used and acceptable. On the outside of the actual muscle cell sits that last layer of connective tissue called the endomysium. The endomysium surrounds the skeletal muscle cell separating it from other muscle cells nestled in the same fascicle. Underneath the endomysium is the cell membrane of this muscle cell/fiber itself, we call it the sarcolemma (means: muscle covering).

Figure 1. Skeletal Muscle Organization. Image thoroughly described below.
Figure 1 Long Description
Within the sarcolemma are all of the cellular components in the sarcoplasm, but what stands out about these cells are that the majority of the cell is made of more bundles. The nuclei are pushed to the outside of the cell while the mitochondria are squeezed into available space between the bundles. These bundles are called myofibrils and are collections of proteins highly organized into groups called sarcomeres. Sarcomeres are the functional unit of skeletal muscle – meaning they are the smallest unit that can perform the functions of a skeletal muscle. Remember that these repeating sarcomeres give the skeletal muscle a striated appearance. We will discuss this more in a later section.
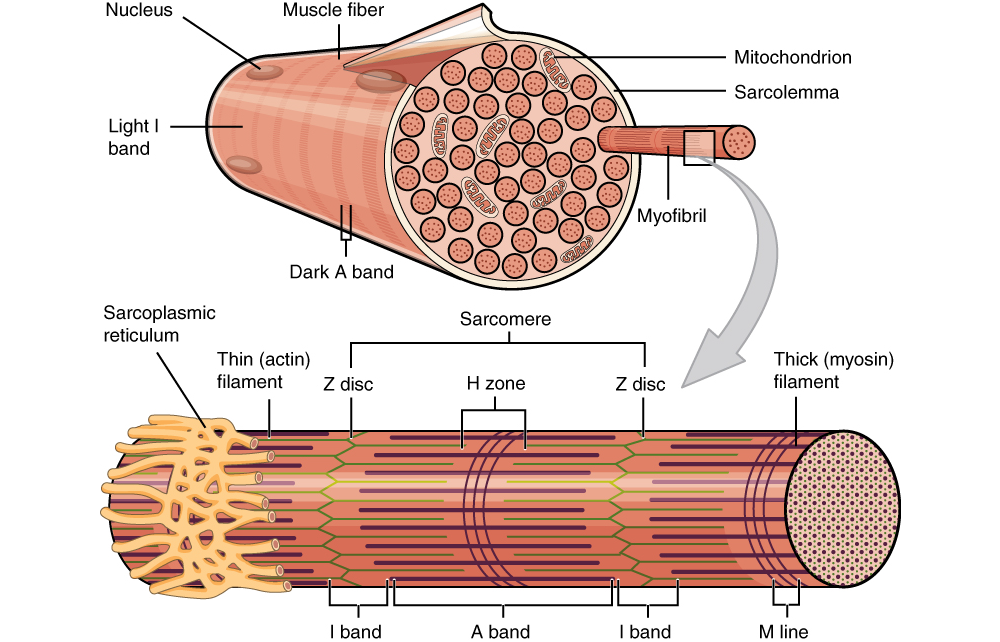
Figure 2. At this point in the module, you are encouraged to pay attention to the right portion of the image depicting a a myofibril protruding from a sarcolemma-surrounded muscle fiber. The myofibril is composed of repeating sarcomere units, depicted in an enlarged form below the grey arrow. Sarcomere compostition will be described in greater detail further in to this module.
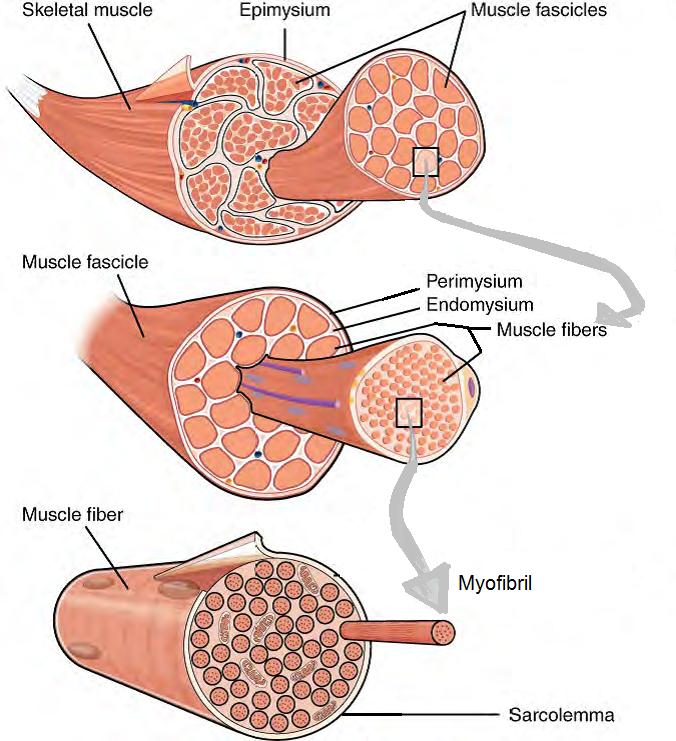
Figure 3. The Three Connective Tissue Layers. The entire skeletal muscle is covered by epimysium. Bundles of muscle fibers, called fascicles, are covered by the perimysium. Individual muscle fibers are covered by the endomysium.
Now that we have deconstructed the muscle, let's reconstruct it! Proteins are organized into repeating sarcomeres and then bundled into myofibrils. Myofibrils are bundled into skeletal muscle cells that are contained by a cell membrane known as the sarcolemma. On top of the skeletal muscle cell is the endomysium. Many of these skeletal muscle cells covered by their individual endomysium are bundled into a fascicle. The fascicle is covered with perimysium. Many fascicles are bundled together to create a whole skeletal muscle. This skeletal muscle is then covered with epimysium. If you are a visual learner seeing this organization in a flow chart may be helpful.
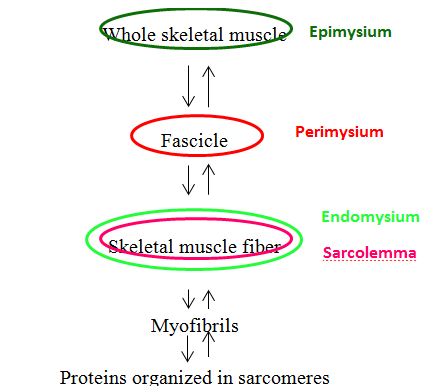
Figure 4. Muscle Fiber Organization Chart. This flow chart depicts the organization of a whole skeletal muscle as it is dissected from whole to its components. The coverings of each layer are denoted by colored circles. The starting point is a whole skeletal muscle covered by a green circle representing as the epimysium. If you were to cut a whole skeletal muscle you would see large groups known as fascicles covered by red circle representing as the perimysium. Looking at each fascicle you will find more groups known as skeletal muscle cells covered by a outer green and an inner pink circle, representing the endomysium and the sarcolemma respectively. Each skeletal muscle cell contains groups known as myofibrils which are composed of sarcomeres. You can reassemble the whole skeletal muscle from sarcomeres by reversing the arrow directionality.
Video 1. View the YouTube video Muscle Fiber Organization (opens in new window)
Looking more closely at the skeletal muscle fiber we can see defined repeating units that we know as sarcomeres. The sarcomere is defined by a Z disc on either end; the Z disc is a lattice work of protein filaments that mark the boundary between adjacent sarcomeres. Simply stated, a sarcomere extends from Z disc to Z disc. The center of the sarcomere is designated by the M line (M = middle).
The sarcomere itself is composed of 6 different protein molecules that can be classified into 3 groups based on its job in muscle contraction.
The contractile proteins are the actual molecules that participate in muscle contraction. One of the contractile proteins is called actin or more commonly called the thin filament. Actin filaments are attached to the Z disc at each end of the sarcomere and radiate inward toward the M line. The actin does not connect in the middle it simply points toward the other actin filament. If you look closely at the actin filament you will see that the filament is actually made out of many much smaller spherical actin subunits. Each actin subunit has a binding site on it for the other contractile protein, myosin. The actin subunits are placed in a double helical pattern around a center structural protein, nebulin. We call actin the thin filament due to its small width only composed of 2 strands of actin subunits and 1 nebulin protein.
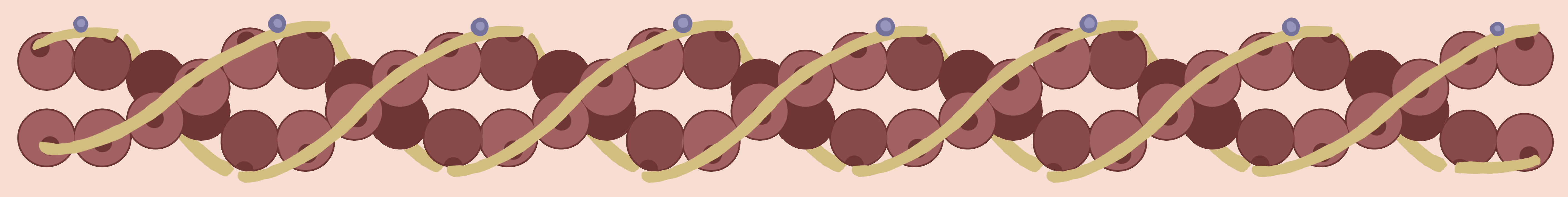
Figure 5. Actin myofilaments contain globular proteins called G-actin molecules (brown balls) that are surrounded by strands of tropomyosin molecules (yellow strands). The troponin protein molecules (blue circles) regulate the movement of the tropomyosin in the presence of calcium.
The second contractile protein is myosin, or more commonly, the thick filament. Myosin filaments are centered at the M line of the sarcomere and extend toward either Z disc without fully reaching them. Titin is the structural protein associated with myosin. The titin is a coiled structural protein that inserts at the Z discs and extends through the middle of the sarcomere. The myosin is anchored to the titin so that it stays unwavering in the middle of the sarcomere when contraction happens instead of shifting in either direction toward a Z disc. If this was to happen damage to the sarcomere would result. If you look closely at the myosin filament you will see it is very thick because the filament is made of many myosin proteins that are wound together. Each individual myosin protein resembles a golf club and has a head portion, a neck/hinge portion, and a tail portion. It is the tail portion of the myosin protein that intertwines with other myosin proteins to create the filament. The head of the myosin protein sticks out of the bundle and can pivot in multiple directions. This head portion is also the part that binds to the binding site on the actin subunit mentioned above.
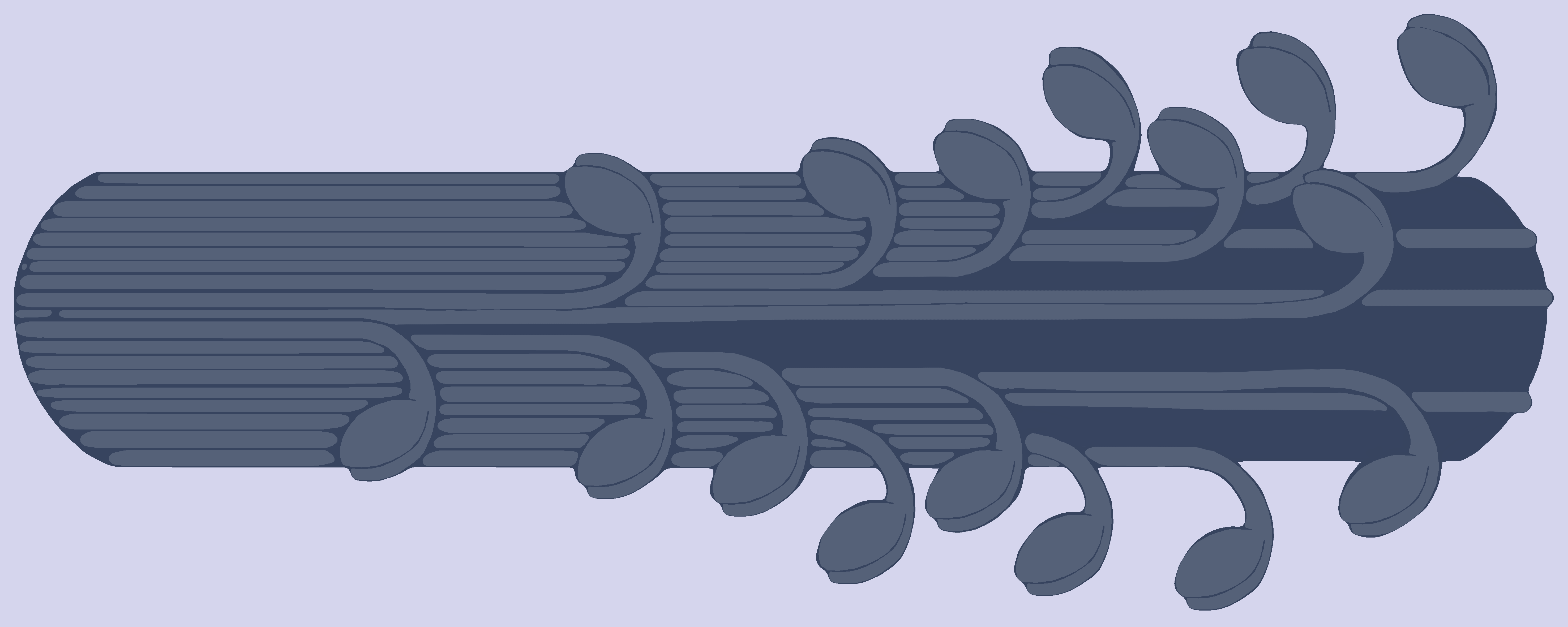
Figure 6. The Myosin filament. Each individual myosin protein resembles a golf club and has a head portion, a neck/hinge portion, and a tail portion. It is the tail portion of the myosin protein that intertwines with other myosin proteins to create the filament (the bundle of golf clubs). The head portion is the part that binds to the myosin binding site on the G-actin protein.
When actin and myosin are arranged in the sarcomere, as seen below, there are various bands and zones that are created. These are labeled and important to know because each band or zone contains a different framework of contractile proteins and therefore refracts light differently. The H zone is located around the M line and extends from one end of the actin filament to the opposing end of the actin filament. This means that the H zone contains only myosin filaments. On an electron micrograph this area appears light grey with a dark line down the center (M line). The I band is located around the Z disc and extends from one end of the myosin filament of one sarcomere to the opposing end of the myosin filament in the NEIGHBORING sarcomere. This means that the I band contains actin filaments only. On an electron micrograph this area appears almost white with a dark line down the center (Z disc). The area is lighter than the H zone because actin is thinner than myosin. The last band is the A band and it extends from one end of the myosin filament of one sarcomere to the opposing end of the myosin filament in the SAME sarcomere. This band is the area of overlap (A = Area of overlap) and contains both actin and myosin filaments. On an electron micrograph this area appears dark grey due to the presence of both filaments with the H zone in the middle of the A band. [Think of the A-band as the d A rk band and the I-band as the l I ght band.] So a sarcomere shows the coloration from one Z disc to the other: white, dark grey, light grey, dark grey, white. If you take your pencil and shade in this pattern what do you see?
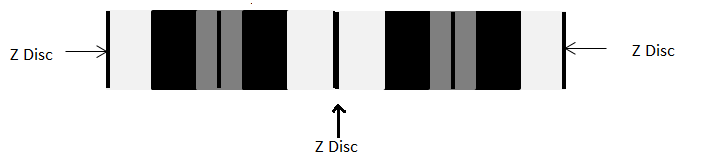
Figure 7. Sarcomere with Z Discs already labeled. In the text below, you will learn about the rest of the bands and zones.
Stripes!!! Remember we call these striations in muscle cells. Also, remember that sarcomeres repeat and if you continue the pattern above the striations become very evident. When the muscle cell is stimulated to contract, the actin is pulled toward the M line by the myosin filaments. It is important to remember that the contractile filaments DO NOT SHORTEN they simply slide past each other toward the middle which shortens the sarcomere, therefore shortening the muscle cell, and fascicle, and whole skeletal muscle. This mode of filament movement is explained later in the Sliding Filament Theory - note how the name describes what takes place.
The question to you is: Which of the bands and zones that we just discussed shorten during contraction?
The correct answer is the H zone and I band. Remember, the A band extends from one end of the myosin filament to the other end of the filament in the SAME sarcomere, and since these thick filaments don't shorten during contraction - the length of this band won't change. However, the H zone consists of myosin only and the I-band of actin only. During contraction the filaments begin to slide and overlap more which means the areas where only one type of filament exists shortens substantially. The way I have always remembered this was that "HI" is a shorter version of "Hello" therefore during contraction the H and I shorten.
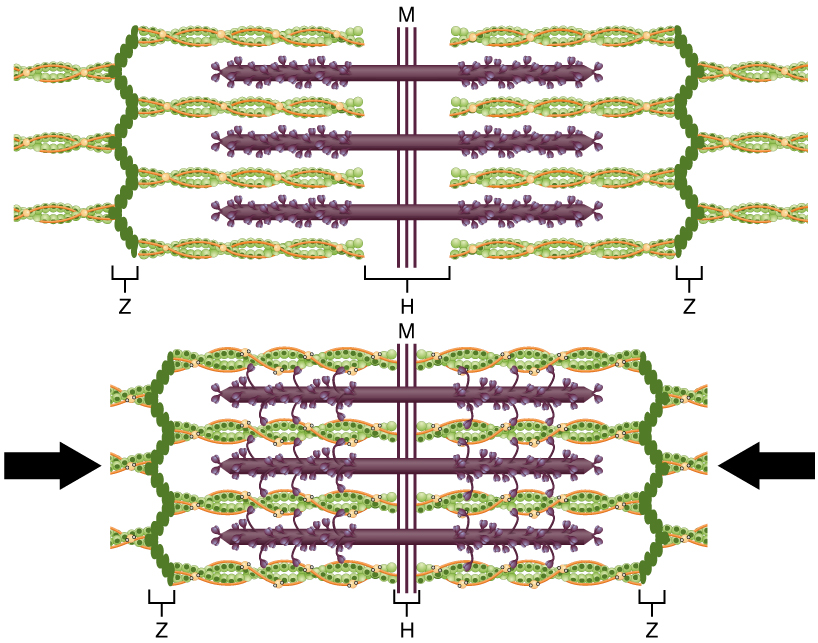
Figure 8. The Sliding Filament Model of Muscle Contraction. When a sarcomere contracts, the Z lines (depicted here by vertical pleated chains of dark green circles) move closer together, and the I band becomes smaller. The A band (shown here as the most contracted width of the H-zone) stays the same. At full contraction, the thin (shown here as twisted green and orange strands) and thick filaments (shown here as purple strands with protrustions) overlap.
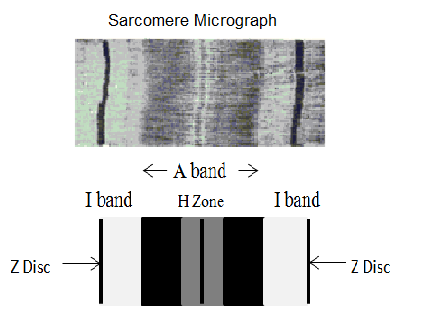
Figure 9. The top image is of an actuall saromere micrograph, while the bottom image provides helpful labels of the (outermost, darkest) Z Disc; (lighter, inside) I band and the (central) A band which encompasses the (lighter) H Zone in the very center. More detailed written descriptions of all of these terms can be found in the glossary.
Video 2. View the YouTube video Sarcomere Analogy (opens in new window)


Before we move on to seeing these proteins in action we must discuss the very important aspect of muscle contraction… being able to turn it on and off. Take a deep breath in, now blow it out. You are able to do that because you turned on the contraction of your diaphragm to take the breath in, and then turned it off to blow the air out by relaxing the diaphragm muscle. If you are unable to turn contraction on and off, muscles may contract and stay that way until they eventually fatigue.You can see the negative implications of that with the deep breath we just took.
So how do we turn contraction on and off? We use regulatory proteins troponin and tropomyosin, both of which are located on the actin (thin) filament. Tropomyosin is a rope like structure that overlay the myosin binding sites on the actin subunits. By blocking the binding sites myosin cannot bind to actin even though they are in close proximity to one another, and the sarcomere will not begin sliding to shorten. I think of it as a door that is closed between actin and myosin. They cannot bind and perform contraction if the closed door (tropomyosin) is in place. The other regulatory protein is troponin, which is composed of 3 subunits. One of the subunits binds to tropomyosin to connect the 2 regulatory proteins together, and another of the subunits binds to calcium. When the muscle is stimulated to contract, calcium is released from its storage facility - the sarcoplasmic reticulum - into the sarcoplasm of the cell. As the calcium travels through the cell it will encounter and bind to troponin. I think of calcium as the key (just the right key, or ion, to unlock the door – not Na or K, etc.) and troponin as the lock on the door, since the two regulatory proteins are attached. When the calcium ion "key" binds with troponin "the lock," it changes the shape of the troponin protein and causes it to rotate, just like turning a key in a lock. As it rotates it pulls tropomyosin off of the binding sites or "opens the door"– remember the 2 regulatory proteins are attached so when one moves so does the other. With tropomyosin out of the way the myosin can reach the actin and start the process of contraction.
Simply put, here is a summary of how to turn contraction on:
1. Calcium is released from the sarcoplasmic reticulum
2. Calcium binds to troponin
3. Troponin moves and pulls tropomyosin off of the myosin binding sites "moving the door"
4. With the binding sites now available the steps of the Sliding Filament Theory begin and contraction occurs.
Now that we can clearly see calcium is the signal to turn contraction on we can extrapolate that when calcium leaves the contraction is turned off. This happens by simply having the opposite of the steps listed above.
To turn contraction off:
1. Calcium is actively pumped back into the sarcoplasmic reticulum and "returns to storage" (requiring the presence of ATP, right?)
2. Calcium (key) is no longer binding with troponin (the lock)
3. Troponin moves and tropomyosin rolls back onto the myosin binding sites "closing the door"
4. With the binding sites no longer available (the door is closed over the opening again) the steps of the Sliding Filament Theory end and contraction ceases.
Understanding this process while making part of contraction clearer still leaves us with many questions. How does the calcium get released? What is the signal for this to occur? Where does this signal come from? We will address these questions in our next segment about the neuromuscular junction.
Video 3. Calcium Troponin Tropomyosin Interaction - "Key Lock Door" Analogy (opens YouTube in new window)
Video 4. Sliding Filament Model (opens Youtube in new window)



Except where otherwise noted, this work by The Community College Consortium for Bioscience Credentials is licensed under a Creative Commons Attribution 4.0 International License.
Text from BioBook licensed under CC BY NC SA and Boundless Biology Open Textbook licensed under CC BY SA.
Other text from OpenStaxCollege licensed under CC BY 3.0. Modified by Alice Rudolph, M.A. and Andrea Doub, M.S. for c3bc.
Instructional Design by Courtney A. Harrington, Ph.D., Helen Dollyhite, M.A. and Caroline Smith, M.A. for c3bc.
Media by Brittany Clark, Jose DeCastro, Jordan Campbell and Antonio Davis for c3bc.
This product was funded by a grant awarded by the U.S. Department of Labor's Employment and Training Administration. The product was created by the grantee and does not necessarily reflect the official position of the U.S. Department of Labor. The Department of Labor makes no guarantees, warranties, or assurances of any kind, express or implied, with respect to such information, including any information on linked sites and including, but not limited to, accuracy of the information or its completeness, timeliness, usefulness, adequacy, continued availability, or ownership.