
Energy, Metabolism and Enzymes
Anatomy & Physiology I
In the human body, potential energy is stored in the bonds between atoms and molecules. Chemical energy is the form of potential energy in which energy is stored in chemical bonds. When those bonds are formed, chemical energy is invested, and when they break, chemical energy is released. Notice that chemical energy, like all energy, is neither created nor destroyed; rather, it is converted from one form to another. When you eat an energy bar before heading out the door for a hike, the honey, nuts, and other foods the bar contains are broken down and rearranged by your body into molecules that your muscle cells convert to kinetic energy.

One characteristic of a living organism is metabolism, which is the sum total of all of the chemical reactions that go on to maintain that organism's health and life. The bonding processes you have learned thus far are anabolic chemical reactions; that is, they form larger molecules from smaller molecules or atoms. But recall that metabolism can proceed in another direction: in catabolic chemical reactions, bonds between components of larger molecules break, releasing smaller molecules or atoms. Both types of reaction involve exchanges not only of matter, but of energy.
Consider the metabolism of sugar. This is a classic example of one of the many cellular processes that use and produce energy. Living things consume sugars as a major energy source, because sugar molecules have a great deal of energy stored within their bonds. For the most part, photosynthesizing organisms like plants produce these sugars. During photosynthesis, plants use energy (originally from sunlight) to convert carbon dioxide gas (CO2) into sugar molecules (like glucose: C6H12O6 ). They consume carbon dioxide and produce oxygen as a waste product. This reaction is summarized as:
Because this process involves synthesizing an energy-storing molecule, it requires energy input to proceed. During the light reactions of photosynthesis, energy is provided by a molecule called adenosine triphosphate (ATP), which is the primary energy currency of all cells. Just as the dollar is used as currency to buy goods, cells use molecules of ATP as energy currency to perform immediate work. In contrast, energy-storage molecules such as glucose are consumed only to be broken down to use their energy. The reaction that harvests the energy of a sugar molecule in cells requiring oxygen to survive can be summarized by the reverse reaction to photosynthesis. In this reaction, oxygen is consumed and carbon dioxide is released as a waste product. The reaction called Cellular Respiration is summarized as:
Both of these reactions involve many steps.
The processes of making and breaking down sugar molecules illustrate two examples of metabolic pathways. A metabolic pathway is a series of chemical reactions that takes a starting molecule and modifies it, step-by-step, through a series of metabolic intermediates, eventually yielding a final product. In the example of sugar metabolism, the first metabolic pathway synthesized sugar from smaller molecules, and the other pathway broke sugar down into smaller molecules. These two opposite processes—the first requiring energy and the second producing energy—are referred to as anabolic pathways (building polymers), and, catabolic pathways (breaking down polymers into their monomers), respectively. Consequently, metabolism is composed of synthesis (anabolism) and degradation (catabolism).
It is important to know that the chemical reactions of metabolic pathways do not take place on their own. Each reaction step is facilitated, or catalyzed, by a protein called an enzyme. Enzymes are important for catalyzing all types of biological reactions—those that require energy as well as those that release energy.
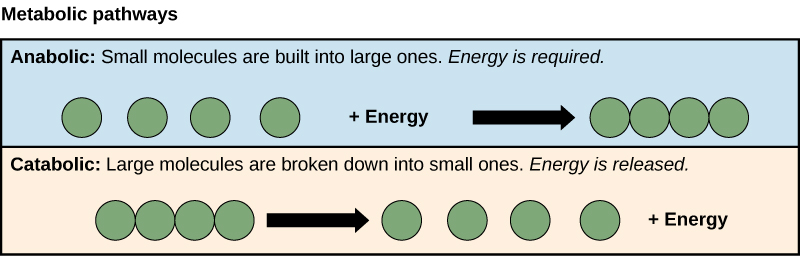
Figure 1. Catabolic pathways, shown in the bottom light brown box, are those that generate energy by breaking down larger molecules. To represent this, four green circles together form a large molecule, which is then broken down into four individual molecules and a release of energy. Anabolic pathways, shown in the top blue box, are those that require energy to synthesize larger molecules. To show this, four individual green circles and energy are combined to form the larger molecule. Both types of pathways are required for maintain the cell's energy balance.
CCBY: OpenStax College
Most processes that occur in the human body are not consciously controlled. They occur continuously to build, maintain, and sustain life. These processes include: organization, in terms of the maintenance of essential body boundaries;metabolism, including energy transfer via anabolic and catabolic reactions; responsiveness; movement; renewal, etc. The cell has to undergo metabolism in order for the tissues, and therefore the organs, to function properly. Energy in its many forms, especially as ATP in our bodies, is necessary to sustain these metabolic processes. Problems with our bodies are actually cellular problems, quite often, so you will need to understand the cell, its needs and its functions, including energy needs and metabolic processes.
View this Metabolic Process Location animation (opens in new window) to learn more about metabolic processes. What kind of catabolism occurs in the heart?
Enzymes catalyze chemical reactions by acting upon another molecule, the reactant or "substrate(s)". In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or more products, which are then released from the active site. The active site is now free to accept another substrate molecule. In the case of more than one substrate, these may bind in a particular order to the active site, before reacting together to produce products.
The general equation representing an enzyme-substrate reaction is:
Substrate + Enzyme → Substrate — Enzyme Complex → Enzyme — Product Complex → Product + Enzyme
It is important to note that the enzyme is unchanged by the reaction. By increasing the substrate concentration, the rate of reaction will increase due to the likelihood of increase in the number of enzyme-substrate complexes; this occurs until the enzyme concentration becomes the limiting factor.
Catalysts cannot make a reaction happen that would not happen on its own. However, they can make reactions happen under less extreme conditions, or help reactions occur that would take extremely long time without a catalyst. Cells must perform and control many reactions, most of which require catalysts of some kind. For most of these reactions, cells use specialized proteins called enzymes as the catalyst. (For now the details of how enzymes catalyze reactions are not important; just understand that enzymes are cellular catalysts.)
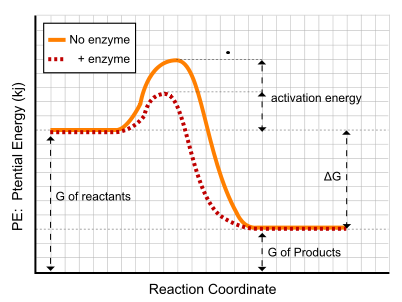
Figure 2. In this diagram, you are shown the energy needed for a reaction to occur with no enzyme (the orange line) verses the energy needed for the reaction when an enzyme is used as a catalyst (the red dashed line). Note that the activation energy is lower when an enzyme is used as a catalyst.
Within cells, the enzymes function is to accelerate various biochemical reactions. For example, sucrose hydrolysis is sped up by an enzyme.
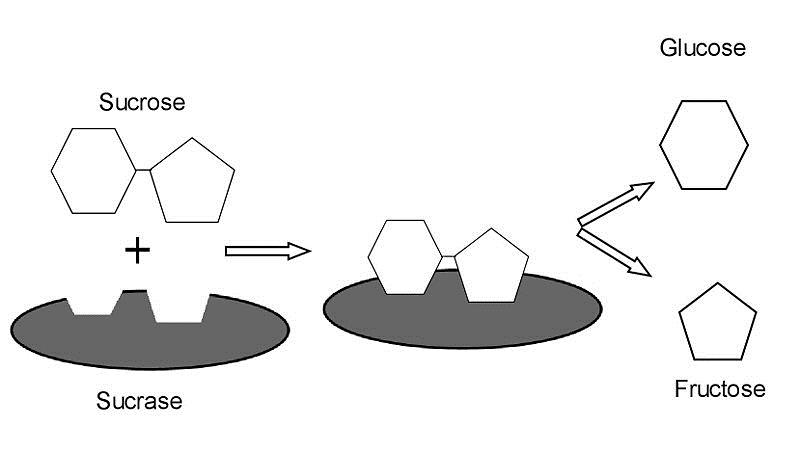
Figure 3. In this example, the hydrolysis of sucrose, C12H22O11, is catalyzed by the enzyme sucrase (the grey oval). The enzyme acts only to accelerate the breakdown of sucrose into glucose, C6H12O6, and fructose, C6H12O6.
CC BY SA: Clarie Hollenbeck, PhD and the Department of Nutriction and Food Science at San Jose State University.
Although spontaneous reactions occur without the input of energy, the rate of the reaction may be very slow. Enzymes speed up the rate of a reaction by acting as a catalyst. Most enzymes are proteins, but some RNA molecules can also perform catalytic functions. Each enzyme is characterized by its high specificity to a particular substrate. Some enzymes can couple thermodynamically favorable and unfavorable reactions.
Virtually all enzymes are proteins, but not all proteins are enzymes and other molecules such as RNA can also catalyze reactions. The most remarkable characteristics of enzymes are their ability to accelerate chemical reactions and their specificity for a particular substrate. Enzymes take advantage of the full range of intermolecular forces, (van der Waals interactions, polar interactions, hydrophobic interactions, and hydrogen bonding), to bring substrates together in most optimal orientation so that reaction will occur.
To work correctly, enzyme molecules must have a very specific shape. That shape is determined by the amino acid sequence in the protein, and the shape forms when the proteins folds up after translation. As an enzyme folds, it forms an active site where chemical substrate(s) fit.
The induced fit model describes how scientists think many enzymes catalyze chemical reactions. Whenever a substrate binds to the active site, the folded shape of the enzyme changes shape slightly, to bind the substrate more tightly. The enzyme tries to shift back to its original shape, which puts strain on chemical bonds in the substrate. These strained chemical bonds undergo chemical reaction more readily. Below are two descriptions of the induced fit model:
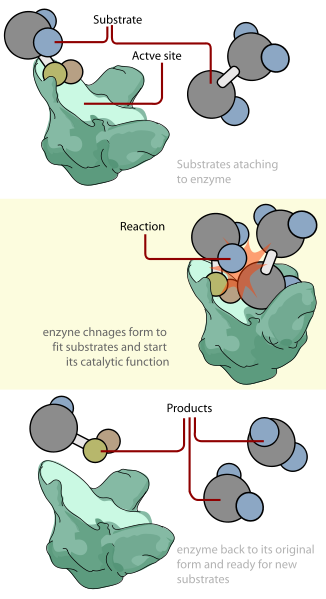
Figure 4. In this diagram, when the enzyme (green) and two substrates (one shown as a large grey sphere with two small blue spheres, a small yellow, and small orange sphere attached and one shown as two large grey spheres with 3 small blue spheres attached) bind there is a slight shift in the enzymes shape to confirm ideal binding. Once the enzyme-substrate complex is formed, it lowers the activation energy and catalyzes the reaction, represented by the orange star shape in the middle image. When the reaction is complete the enzyme shape shifts back therefore releasing the three products (two products are large grey spheres with two small blue spheres attached and one product is a large grey sphere with a small blue, yellow, and orange sphere attached).
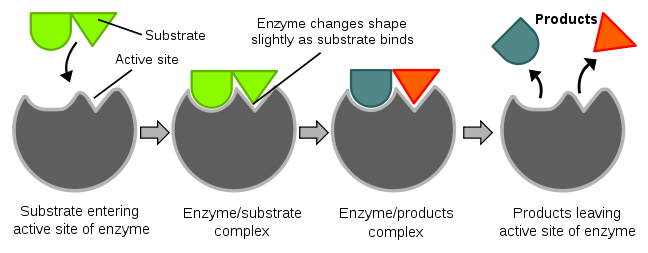
Figure 5. In this diagram, when the enzyme (grey) and substrate (bright green) bind there is a slight shift in the enzymes shape to confirm ideal binding. Once the enzyme-substrate complex is formed, it lowers the activation energy and catalyzes the reaction. When the reaction is complete the enzyme shape shifts back therefore releasing the products (dark teal and orange).
Most enzymes have an active site that is substrate specific, meaning the active site can bind and catalyze a reaction for one or a few different substrates. Substrate specificity depends on the shape of the active site where the substrate binds. If the shape of the active site changes slightly, the substrate may not bind anymore. And even if the substrate is able to bind, an enzyme with an altered shape may not be able to carry out its normal function of catalyzing a specific chemical reaction.
Enzyme inhibitors are small molecules that bind directly to enzyme molecules and change the shape of the enzyme in some way, which slows or stops the enzyme from converting substrates into products. Inhibitors work in several different ways.
Competitive inhibitors are molecules that fit in the enzyme binding site just like the normal substrate. If an enzyme is added to a mixture of the normal substrate and the inhibitor, some enzyme molecules will bind the substrate and others the inhibitor. Since the two molecules are competing for the enzyme molecules, whichever molecule is in the highest concentration usually binds most often.
Competitive inhibitor molecules do not undergo a chemical reaction, (bind and change the shape of the enzyme), only normal substrate molecules do. The net result of competitive inhibition is that less product is made.
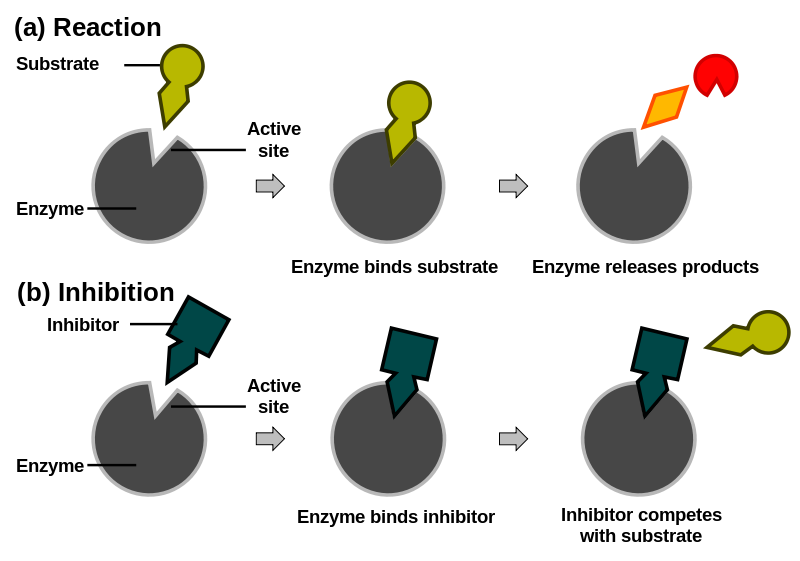
Figure 6. In this generalized diagram the top example shows how a substrate (olive green) typically binds to an enzyme (grey) at the active site. The reaction proceeds and the products (orange) are released. In the bottom panel, a competitor molecule (dark green) is in the active site normally occupied by the natural substrate therefore inhibiting the reaction.
Many important competitive inhibitors of enzymes have been discovered. For example, sulfonamide was one of the first commercial drugs for treating bacterial infections. Bacteria use the enzyme dihydropteroate synthetase, (DHPS), to convert 4-aminobenzoic acid to folic acid, an essential coenzyme. Sulfonamide binds to the same active site on DHPS as the normal substrate, and competitively inhibits the enzyme. As a result of this process of inhibition, the bacteria are "starved" of folate and die. Non-competitive inhibitors are another type of reversible inhibitors. They are molecules that bind to enzyme molecules somewhere other than the main substrate binding site. If enzyme is added to a mixture of the normal substrate and non-competitive inhibitor, all of the binding sites on the enzyme molecules will be available to bind the substrate. However, the inhibitor molecules attach to another site and slow down enzyme activity. Once again, fewer products are made.
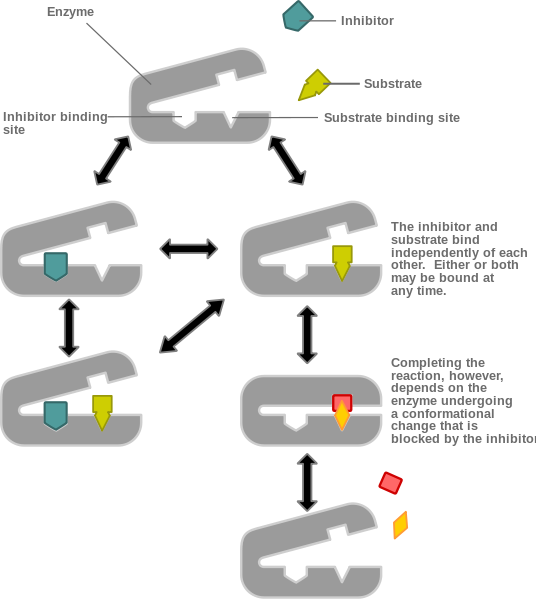
Figure 7. A non-competitive inhibitor. The above diagram shows non-competitive inhibition. Here inhibitor molecules (teal) bind to sites other than the active binding site of the main substrate (yellow). The inhibitor (teal) and substrate (yellow) bind independently and either or both may be bound at any time. When an inhibitor molecule (teal) binds to the enzyme (grey), such as in the left pathway, it prevents the enzyme (grey) from undergoing the conformational change needed to complete the reaction with the substrate (yellow). The non-inhibited reaction is shown on the right where the enzyme (grey) binds with the substrate (yellow) and completes the reaction then releasing two products, shown here as an orange diamond and a red square.
This video discusses enzymes from a macromolecule and metabolic perspective:
Video 1. Enzymes: A Macromolecule Perspective video (opens Youtube in new window)


Except where otherwise noted, this work by The Community College Consortium for Bioscience Credentials is licensed under a Creative Commons Attribution 4.0 International License.
Text from BioBook licensed under CC BY NC SA and Boundless Biology Open Textbook licensed under CC BY SA.
Other text from OpenStaxCollege licensed under CC BY 3.0. Modified by Alice Rudolph, M.A. for c3bc.
Instructional Design by Courtney A. Harrington, Ph.D., Helen Dollyhite, M.A. and Caroline Smith, M.A. for c3bc.
Media by Brittany Clark and Antonio Davis for c3bc
This product was funded by a grant awarded by the U.S. Department of Labor's Employment and Training Administration. The product was created by the grantee and does not necessarily reflect the official position of the U.S. Department of Labor. The Department of Labor makes no guarantees, warranties, or assurances of any kind, express or implied, with respect to such information, including any information on linked sites and including, but not limited to, accuracy of the information or its completeness, timeliness, usefulness, adequacy, continued availability, or ownership.