
Chemical Bonds, Reactions and Vital Inorganic Compounds
Anatomy & Physiology I
An atom is considered an ion when it gains or loses one or more electrons, and therefore carries a charge. These oppositely charged atoms, or ions, are attracted to one another and form ionic bonds. An ionic bond can occur between any cation, (a positively charged ion), and anion, (a negatively charged ion).
The following video discusses the formation of ions and how ionic compounds are made.
Video 1. View The Formation of Ions video on YouTube (opens in a new window).

Covalent bonds form when two atoms share pairs of electrons to achieve the octet rule. The shared electrons orbit the nuclei of both the bonding atoms; the overlap of electron clouds is the bond. Covalently bonded atoms form discrete particles known as molecules. A molecule is a group of two or more atoms held together by covalent bonding. A molecule will always have a set number of atoms. For example, in a water molecule, one atom of oxygen forms 2 covalent bonds with 2 Hydrogen atoms. Thus each water molecule (H2O) has exactly 1 atom of Oxygen and 2 atoms of Hydrogen.
In some cases it is necessary for two atoms to share more than just one pair of electrons between them to achieve an octet. If one pair of electrons (2 electrons) is shared between two atoms it is called a single bond. If two pairs of electrons (4 electrons) are shared between two atoms, it is called a double bond. If three pairs of electrons (6 electrons) are shared between two atoms, it is called a triple bond.
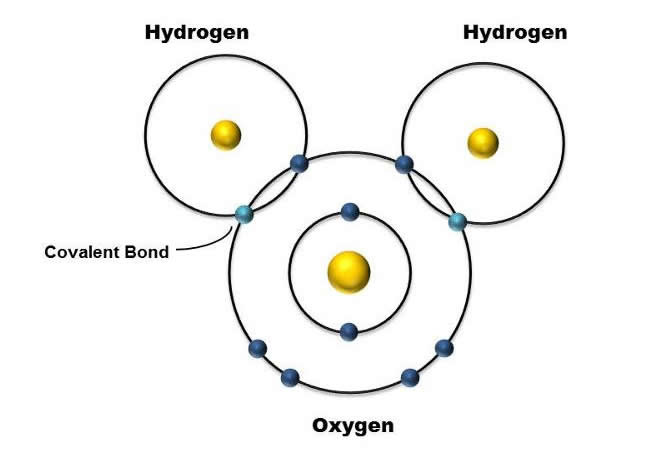
Figure 1.Covalent Bonds of H2O. The protons, represented by a yellow ball, are surrounded by blue electron balls. The two lighter blue shaded electrons represent the two electrons shared between oxygen and hydrogen.
There are two types of covalent bonds: polar and nonpolar. In a polar covalent bond, the electrons are unequally shared by the atoms because they are more attracted to one nucleus than the other. The relative attraction of an atom to an electron is known as its electronegativity: atoms that are more attracted to an electron are considered to be more electronegative Because of the unequal distribution of electrons between the atoms of different elements, a slightly positive (δ+) or slightly negative (δ–) charge develops. This partial charge is known as a dipole; this is an important property of water and accounts for many of its characteristics. The dipole in water occurs because oxygen has a higher electronegativity than hydrogen, which means that the shared electrons spend more time in the vicinity of the oxygen nucleus than they do near the nucleus of the hydrogen atoms.
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. For example, molecular oxygen (O2) is nonpolar because the electrons will be equally distributed between the two oxygen atoms. The four bonds of methane are also considered to be nonpolar because the electronegativities of carbon and hydrogen are nearly identical.

Not all bonds are ionic or covalent; weaker bonds can also form between molecules. Two types of weak bonds that frequently occur are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist.
Hydrogen bonds occur when there is an attraction between two polar molecules, a hydrogen atom and an electronegative atom (e.g. nitrogen, oxygen). Polar molecules, in this instance, are molecules in which the electrons are not shared equally among covalently bonded atoms, resulting in part of the molecule being slightly positive and the other being slightly negative. The figure below illustrates the interaction of hydrogen bonding in water.
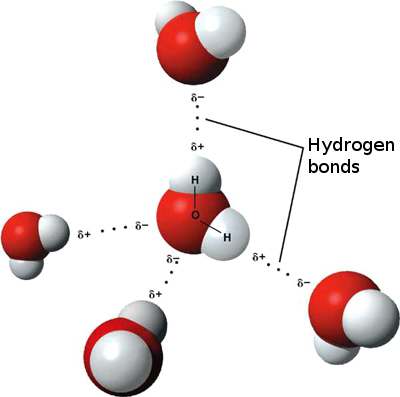
Figure 2.: Hydrogen bonds between water molecules. The two white hydrogen atoms are covalently bonded to the red oxygen atom. Dotted lines show the hydrogen bonds between the white hydrogens and the red oxygen of a neighboring molecule.
CC BY SA: Michael Manas
Like hydrogen bonds, Van der Waals interactions are weak interactions between molecules. Van der Waals attractions can occur between any two or more molecules and are dependent on slight fluctuations of the electron densities, which can lead to slight temporary dipoles around a molecule. For these attractions to happen, the molecules need to be very close to one another. These interactions are said to be responsible for geckos ability to cling to and climb glass as seen in the figure below.


One characteristic of a living organism is metabolism, which is the sum total of all of the chemical reactions that go on to maintain that organism's health and life. The bonding processes you have learned thus far are anabolic chemical reactions; that is, they form larger molecules from smaller molecules or atoms. But recall that metabolism can proceed in another direction: in catabolic chemical reactions, bonds between components of larger molecules break, releasing smaller molecules or atoms. Both types of reaction involve exchanges not only of matter, but of energy.
Chemical reactions require a sufficient amount of energy to cause the matter to collide with enough precision and force that old chemical bonds can be broken and new ones formed. In general, kinetic energy is the form of energy powering any type of matter in motion. Imagine you are building a brick wall. The energy it takes to lift and place one brick atop another is kinetic energy, the energy matter possesses because of its motion. Once the wall is in place, it stores potential energy. Potential energy is the energy of position, or the energy matter possesses because of the positioning or structure of its components. If the brick wall collapses, the stored potential energy is released as kinetic energy as the bricks fall.
In the human body, potential energy is stored in the bonds between atoms and molecules. Chemical energy is the form of potential energy in which energy is stored in chemical bonds. When those bonds are formed, chemical energy is invested, and when they break, chemical energy is released. Notice that chemical energy, like all energy, is neither created nor destroyed; rather, it is converted from one form to another. When you eat an energy bar before heading out the door for a hike, the honey, nuts, and other foods the bar contains are broken down and rearranged by your body into molecules that your muscle cells convert to kinetic energy.
Chemical reactions that release more energy than they absorb are characterized as exergonic. The catabolism of the foods in your energy bar is an example. Some of the chemical energy stored in the bar is absorbed into molecules your body uses for fuel, but some of it is released, for example, as heat. In contrast, chemical reactions that absorb more energy than they release are endergonic. These reactions require energy input, and the resulting molecule stores not only the chemical energy in the original components, but also the energy that fueled the reaction. Because energy is neither created nor destroyed, where does the energy needed for endergonic reactions come from? In many cases, it comes from exergonic reactions.
You have already learned that chemical energy is absorbed, stored, and released by chemical bonds. In addition to chemical energy, mechanical, radiant, and electrical energy are important in human functioning.
An inorganic compound is a substance that does not contain both carbon and hydrogen. A great many inorganic compounds do contain hydrogen atoms, such as water (H2O) and the hydrochloric acid (HCl) produced by your stomach. In contrast, only a handful of inorganic compounds contain carbon atoms. Carbon dioxide (CO2) is one of the few examples.
Water molecules tend to stick together as a result of hydrogen bonding. This creates surface tension which makes water form droplets and allows for organisms like insects to walk on water. Cohesion is also responsible for water's ability to move against gravity from the roots of a tree to its leaves, trees could not survive without this ability. Without trees, there wouldn't be enough oxygen for other life on Earth.
The animation below illustrates cohesion and surface tension.
Animation 1. View the Cohesion of Water animation on YouTube (opens in new window).
Cohesion allows for the development of surface tension, the capacity of a substance to withstand being ruptured when placed under tension or stress. This is also why water forms droplets when placed on a dry surface rather than being flattened out by gravity. When a small scrap of paper is placed onto the droplet of water, the paper floats on top of the water droplet even though paper is denser (heavier) than the water. Cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. It's even possible to float a needle on top of a glass of water if it is placed gently without breaking the surface tension.
The following video demonstrates surface tension:
Video 2: Click to view the Surface Tension video on YouTube (opens in new window).
Cohesion is also related to water's property of adhesion, or the attraction between water molecules and other molecules. This attraction is sometimes stronger than water's cohesive forces, especially when the water is exposed to charged surfaces such as those found on the inside of thin glass tubes known as capillary tubes. Adhesion is observed when water "climbs" up the tube placed in a glass of water: notice that the water appears to be higher on the sides of the tube than in the middle. This is because the water molecules are attracted to the charged glass walls of the capillary more than they are to each other and, therefore, adhere to it. This type of adhesion is the called capillary action.
The image below illustrates water adhering to the side of the glass tube forming a meniscus.
Figure 3. Meniscus of water in a graduated cylinder. The water appears to be higher on the sides than in the middle, since the water molecules are attracted to the glass.The water is measured at the bottom of the meniscus, so it is 21mL.
Since water consists of polar molecules, it is able to dissolve many substances into a uniform mixture or solution. The water molecules surround the molecules of the solute, making the water the solvent. Cells and organisms depend on the polarity of water. They are composed of atoms and molecules that dissolve in water to form different aqueous solutions. These solutions are separated into different compartments by layers of nonpolar lipids, which don't dissolve in water. Without this difference, cells could not create specialized areas for different functions, which is one of the most fundamental properties of cells.
Play the animation below that illustrates universal solvency of water.
Animation 2. Click to view the Universal Solvency of Water animation on YouTube (opens in a new window).
Water is an example of a heat sink. A heat sink is a substance or object that absorbs and dissipates heat but does not experience a corresponding increase in temperature. In the body, water absorbs the heat generated by chemical reactions without greatly increasing in temperature. Moreover, when the environmental temperature soars, the water stored in the body helps keep the body cool. This cooling effect happens as warm blood from the body's core flows to the blood vessels just under the skin and is transferred to the environment. At the same time, sweat glands release warm water in sweat. As the water evaporates into the air, it carries away heat, and then the cooler blood from the periphery circulates back to the body core. Temperature is a measure of the average movement of molecules, in water, when we first add heat, there is no rise in temperature because the energy is breaking bonds. Only once, the bonds are broken and the energy start increasing the movement of the water molecules does the temperature rise. Water's unique ability to absorb heat is due to it's relatively high specific heat.
Various mixtures of solutes and water are described in chemistry. The concentration of a given solute is the number of particles of that solute in a given space (oxygen makes up about 21 percent of atmospheric air). In the bloodstream of humans, glucose concentration is usually measured in milligram (mg) per deciliter (dL), and in a healthy adult averages about 100 mg/dL. Another method of measuring the concentration of a solute is by its molarilty—which is moles (M) of the molecules per liter (L). The mole of an element is its atomic weight, while a mole of a compound is the sum of the atomic weights of its components, called the molecular weight. An often-used example is calculating a mole of glucose, with the chemical formula C6H12O6. Using the periodic table, the atomic weight of carbon (C) is 12.011 grams (g), and there are six carbons in glucose, for a total atomic weight of 72.066 g. Doing the same calculations for hydrogen (H) and oxygen (O), the molecular weight equals 180.156g (the "gram molecular weight" of glucose). When water is added to make one liter of solution, you have one mole (1M) of glucose. This is particularly useful in chemistry because of the relationship of moles to "Avogadro's number." A mole of any solution has the same number of particles in it: 6.02 × 1023. Many substances in the bloodstream and other tissue of the body are measured in thousandths of a mole, or millimoles (mM). A colloid is a mixture that is somewhat like a heavy solution. The solute particles consist of tiny clumps of molecules large enough to make the liquid mixture opaque (because the particles are large enough to scatter light). Familiar examples of colloids are milk and cream. In the thyroid glands, the thyroid hormone is stored as a thick protein mixture also called a colloid. A suspension is a liquid mixture in which a heavier substance is suspended temporarily in a liquid, but over time, settles out. This separation of particles from a suspension is called sedimentation. An example of sedimentation occurs in the blood test that establishes sedimentation rate, or sed rate. The test measures how quickly red blood cells in a test tube settle out of the watery portion of blood (known as plasma) over a set period of time. Rapid sedimentation of blood cells does not normally happen in the healthy body, but aspects of certain diseases can cause blood cells to clump together, and these heavy clumps of blood cells settle to the bottom of the test tube more quickly than do normal blood cells.
Two types of chemical reactions involve the creation or the consumption of water:
In dehydration synthesis, one reactant gives up an atom of hydrogen and another reactant gives up a hydroxyl group (OH) in the synthesis of a new product. In the formation of their covalent bond, a molecule of water is released as a byproduct (Figure 4). This is also sometimes referred to as a condensation reaction.
In hydrolysis, a molecule of water disrupts a compound, breaking its bonds. The water is itself split into H and OH. One portion of the severed compound then bonds with the hydrogen atom, and the other portion bonds with the hydroxyl group. These reactions are reversible, and play an important role in the chemistry of organic compounds (which will be discussed shortly).
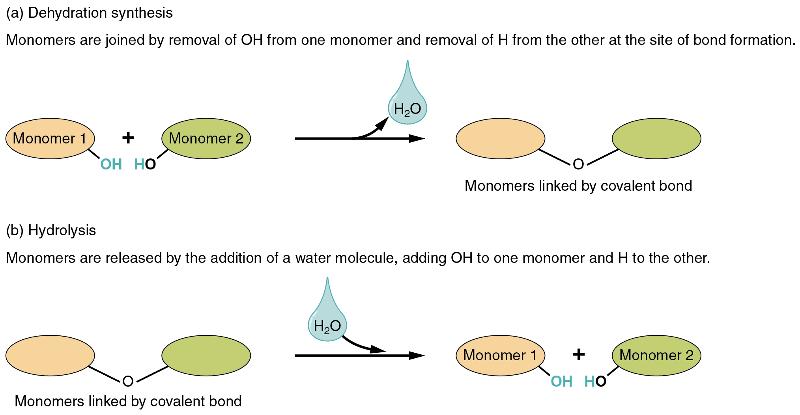
Figure 4 Dehydration Synthesis and Hydrolysis Monomers, the basic units for building larger molecules, form polymers (two or more chemically-bonded monomers). (a) In dehydration synthesis, two monomers are covalently bonded in a reaction in which one gives up a hydroxyl group and the other a hydrogen atom. A molecule of water is released as a byproduct during dehydration reactions. (b) In hydrolysis, the covalent bond between two monomers is split by the addition of a hydrogen atom to one and a hydroxyl group to the other, which requires the contribution of one molecule of water.
CC BY: Openstax College
Recall that salts are formed when ions form ionic bonds. In these reactions, one atom gives up one or more electrons, and thus becomes positively charged, whereas the other accepts one or more electrons and becomes negatively charged. You can now define a salt as a substance that, when dissolved in water, dissociates into ions other than H+ or OH– . This fact is important in distinguishing salts from acids and bases, discussed next. A typical salt, NaCl, dissociates completely in water (Figure 5). The positive and negative regions on the water molecule (the hydrogen and oxygen ends respectively) attract the negative chloride and positive sodium ions, pulling them away from each other. Again, whereas nonpolar and polar covalently bonded compounds break apart into molecules in solution, salts dissociate into ions. These ions are electrolytes; they are capable of conducting an electrical current in solution. This property is critical to the function of ions in transmitting nerve impulses and prompting muscle contraction.
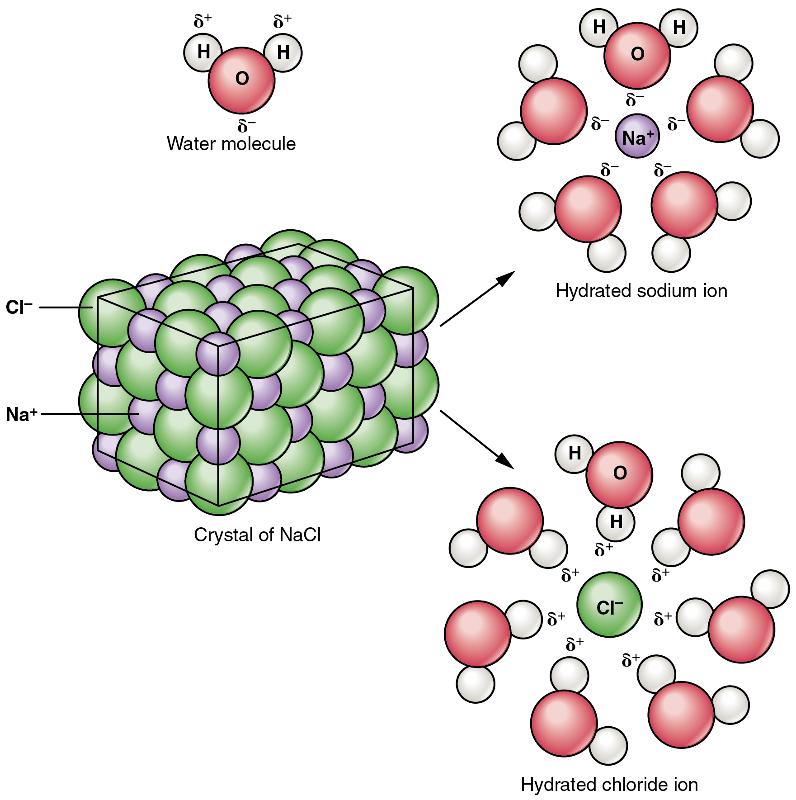
Figure 5 Dissociation of Sodium Chloride in Water: Notice that the crystals of sodium chloride dissociate not into molecules of NaCl, but into Na+ cations and Cl– anions, each completely surrounded by water molecules.
CC BY: Openstax College
Many other salts are important in the body. For example, bile salts produced by the liver help break apart dietary fats, and calcium phosphate salts form the mineral portion of teeth and bones.
The pH of a solution indicates its acidity or alkalinity. Hydrogen ions are generated in pure water by the dissociation (ionization) of a small percentage of water molecules into equal numbers of hydrogen (H+) ions and hydroxide (OH-) ions.
An acid is an inorganic substance that increases the concentration of hydrogen ions (H+) in a solution, usually by having one of its hydrogen atoms dissociate.
A base is an inorganic subsatnce that provides either hydroxide ions (OH - ) or other negatively charged ions that combine with hydrogen ions, reducing their concentration in the solution and thereby raising the pH. In cases where the base releases hydroxide ions, these ions bind to free hydrogen ions, generating new water molecules.
The stronger the acid, the more readily it donates H+. For example, hydrochloric acid (HCl) completely dissociates into hydrogen and chloride ions and is highly acidic, whereas the acids in tomato juice or vinegar do not completely dissociate and are considered weak acids. Conversely, strong bases are those substances that readily donate OH- or take up hydrogen ions. Sodium hydroxide (NaOH) and many household cleaners are highly alkaline and give up OH - rapidly when placed in water, thereby raising the pH. An example of a weak basic solution is seawater, which has a pH near 8.0, close enough to neutral pH that marine organisms adapted to this saline environment are able to thrive in it.
The pH scale is an inverse logarithm and ranges from 0 to 14 ( See pH scale below). Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. Extremes in pH in either direction from 7.0 are usually considered inhospitable to life. The pH inside cells (6.8) and the pH in the blood (7.4) are both very close to neutral. However, the environment in the stomach is highly acidic, with a pH of 1 to 2. So how do the cells of the stomach survive in such an acidic environment? How do they homeostatically maintain the near neutral pH inside them? The answer is that they cannot do it and are constantly dying. New stomach cells are constantly produced to replace dead ones, which are digested by the stomach acids. It is estimated that the lining of the human stomach is completely replaced every seven to ten days.
The pH of most living cells is close to 7. Even a minor change in pH can be catastrophic for a cell because the chemical processes within the cell are very sensitive to hydrogen ion concentration [H+] and hydroxide ion concentration [OH-] fluctuations. To prevent pH changes in blood, a chemical system is in place to help maintain a stable pH. For instance, if a liter of pure water has 0.01 moles of strong acid added to it, the pH will go from 7 to 2. However, if that same amount of acid is added to blood, the pH only goes from 7.4 to 7.3. This resistance to pH change is due to substances called buffers in the blood. Buffers are substances that accept hydrogen ions [H+] when they are in excess, and donate hydroxide ions [OH-] when they are depleted to prevent changes in blood, which has a narrow pH range of 7.35-7.45. This process minimizes the effect of adding an acid or base. Buffers normally contain a weak acid and its corresponding base, which can combine reversibly with [H+].
The following video discusses pH:
Video 3. View the Introduction to pH video on YouTube (opens in new window).
So how can organisms whose bodies require a near-neutral pH ingest acidic and basic substances (a human drinking orange juice, for example) and survive? Buffers are the key. Buffers, solutions or substances that resist changes in pH readily absorb excess H+ or OH -, keeping the pH of the body carefully maintained in the narrow range required for survival. Maintaining a constant blood pH is critical to a person's well-being. The buffer maintaining the pH of human blood involves carbonic acid (H2CO3), bicarbonate ion (HCO3- ), and carbon dioxide (CO2). When bicarbonate ions combine with free hydrogen ions and become carbonic acid, hydrogen ions are removed, moderating pH changes. Similarly, as shown in the figure below, excess carbonic acid can be converted to carbon dioxide gas and exhaled through the lungs. This prevents too many free hydrogen ions from building up in the blood and dangerously reducing the blood's pH. Likewise, if too much OH - is introduced into the system, carbonic acid will combine with it to create bicarbonate, lowering the pH ( H2CO3 + OH- ![]() HCO3 - + H2O) . Without this buffer system, the body's pH would fluctuate enough to put survival in jeopardy.
HCO3 - + H2O) . Without this buffer system, the body's pH would fluctuate enough to put survival in jeopardy.
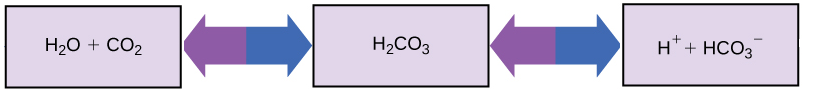
Figure 6. This diagram shows the body's buffering of blood pH levels. The blue arrows represent the process of raising pH as more CO2 is made. The purple arrows indicate the reverse process: the lowering of pH as more bicarbonate is created.
CC BY: Openstax College
Other examples of buffers are antacids used to combat excess stomach acid. Many of these over the-counter medications work in the same way as blood buffers, usually with at least one ion capable of absorbing hydrogen and moderating pH, bringing relief to those that suffer "heartburn" after eating. The unique properties of water that contribute to this capacity to balance pH—as well as water's other characteristics—are essential to sustaining life on Earth.

Acid: inorganic molecule that donates hydrogen ions and increases the concentration of hydrogen ions in a solution.
Adhesion: attraction between water molecules and other molecules.
Anion: A negatively charged ion.
Base: inorganic molecule that donates hydroxide ions or otherwise binds excess hydrogen ions and decreases the concentration of hydrogen ions in a solution.
Buffer: substance that prevents a change in pH by absorbing or releasing hydrogen or hydroxide ions.
Cation: A positively charged ion.
Chemical Energy: form of energy that is absorbed as chemical bonds form, stored as they are maintained, and released as they are broken.
Cohesion: intermolecular forces between water molecules caused by the polar nature of water; responsible for surface tension
Colloid: a mixture that is somewhat like a heavy solution
Covalent Bonds: Form when two atoms share pairs of electrons. The shared electrons orbit the nuclei of both the bonding atoms; the overlap of electron clouds is the bond.
Electrical Energy: supplied by electrolytes in cells and body fluids, contributes to the voltage changes that help transmit impulses in nerve and muscle cells.
Endergonic: Chemical reactions that absorb more energy than they release.
Exergonic: Chemical reactions that release more energy than they absorb.
Heat Sink: a substance or object that absorbs and dissipates heat but does not experience a corresponding increase in temperature.
Hydrogen Bonds: Occur when there is an attraction between two polar molecules, a hydrogen atom and an electronegative atom (e.g. nitrogen, oxygen).
Inorganic Compound: Substance that does not contain both carbon and hydrogen
Kinetic Energy: energy that matter possesses because of its motion.
Mechanical Energy: is stored in physical systems such as machines, engines, or the human body, directly powers the movement of matter.
Nonpolar Covalent Bonds: Form between two atoms of the same element or between different elements that share electrons equally.
pH Scale: Scale ranging from zero to 14 that is inversely proportional to the concentration of hydrogen ions in a solution.
Polar Covalent Bonds: The electrons are unequally shared by the atoms because they are more attracted to one nucleus than the other.
Potential Energy: stored energy matter possesses because of the positioning or structure of its components.
Radiant Energy: is energy emitted and transmitted as waves rather than matter. These waves vary in length. The full spectrum of radiant energy is referred to as the electromagnetic spectrum.
Solute: A substance dissolved in another substance.
Solvent: substance capable of dissolving another substance.
Specific Heat: the amount of heat one gram of a substance must absorb or lose to change its temperature by one degree Celsius.
Surface Tension: tension at the surface of a body of liquid that prevents the molecules from separating; created by the attractive cohesive forces between the molecules of the liquid
Suspension: a liquid mixture in which a heavier substance is suspended temporarily in a liquid, but over time, settles out
Van der Waals Interactions: These weak attractions can occur between any two or more molecules and are dependent on slight fluctuations of the electron densities, which can lead to slight temporary dipoles around a molecule.

Except where otherwise noted, this work by The Community College Consortium for Bioscience Credentials is licensed under a Creative Commons Attribution 3.0 Unported License.
Text from BioBook licensed under CC BY NC SA and Boundless Biology Open Textbook licensed under CC BY SA. Modified by Courtney A. Harrington, Ph.D. for c3bc.
Text also from OpenStaxCollege licensed under CC BY 3.0. Modified by Alice Rudolph, M.A. for c3bc.
Additional text written by Traci Rutledge, M.S. and Kirsten Williford, Ph.D. for c3bc.
Instructional Design by Nicole Lipscomb, M.S., Irene Yee Chief, Ph.D., Courtney Harrington, Ph.D., Helen Dollyhite, M.A. and Caroline Smith, M.A. for c3bc.
Media and interactive objects by Lucious Oliver, II, Joe deCastro, Brittany Clark and John Reece for c3bc.