Fats and Oils
A fat is a large molecule oils are made up of glycerol and fatty acids . Glycerol is an organic compound (alcohol) with three carbons, five hydrogens, and three hydroxyl (OH) groups. Fatty acids have a long chain of hydrocarbons to which a carboxyl group is attached.
The number of carbons in the fatty acid may range from 4 to 36; most common are those containing 12-18 carbons. In a fat molecule, the fatty acids are attached to each of the three carbons of the glycerol molecule with an ester bond through an oxygen atom.
Dehydration synthesis reactions join three fatty acids to the glycerol molecule. The primary function of fats and oils is energy storage. A triglyceride (C55H98O6) is a glycerol with three fatty acids. This is why it is unhealthy to have "high triglycerides". It means that the amount of fat in your blood is too high, which can raise your risk for heart disease.
Outlined below are the difference between fats and oils:
- Fats are usually of animal origin. One commonly known fat is lard, which comes from the fat of a pig. Also, fats solidify of at room temperature. Fats are usually solids that usually have low melting points. Typically, the fatty acids that make up fats are saturated. A saturated fatty acid does not have a double bond between carbon atoms. Due to the lack of a double bond in the molecule, it is considered saturated with hydrogen. This means that it has as many hydrogen ions as it possibly can. Saturated fatty acids are long straight molecules.
- Oils are lipids and usually of plant origin and are typically unsaturated. The oils most familiar to us are things like olive oil, sunflower oil, and peanut oil an do not solidify at room temperature. In an unsaturated fatty acid, there is at least one double bond between carbon atoms. Since an extra electron is shared between the carbons, fewer hydrogen ions can bond, making the molecule unsaturated with hydrogens. Unsaturated fatty acids have bends at the double bonds.
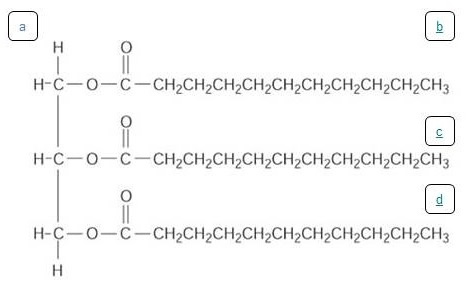
Figure 4. The molecule shown above is a Saturated Fat, where all of the fatty acid carbons have single bonds. The fat has one glycerol molecule (represented by the letter a, located on the left side of the image). Three fatty acid "tails" (represented by the letters b,c, and d,) are placed vertically on the right side of the image).

