Steps of the Sliding Filament Theory
Earlier in the chapter we discussed that when contraction occurs the myosin filaments pull the actin filaments toward the M line of the sarcomere shortening not only the sarcomere but the entire skeletal muscle cell as well. Neither filament changes in length, they simply slide past each other, which is why we refer to the steps of contraction as the Sliding Filament Theory.
Resuming our excitation - contraction coupling saga, once calcium has been released from the sarcoplasmic reticulum, calcium binds to troponin, tropomyosin is moved out of the way, and actin and myosin can now begin to interact and the Sliding Filament Theory ensues.
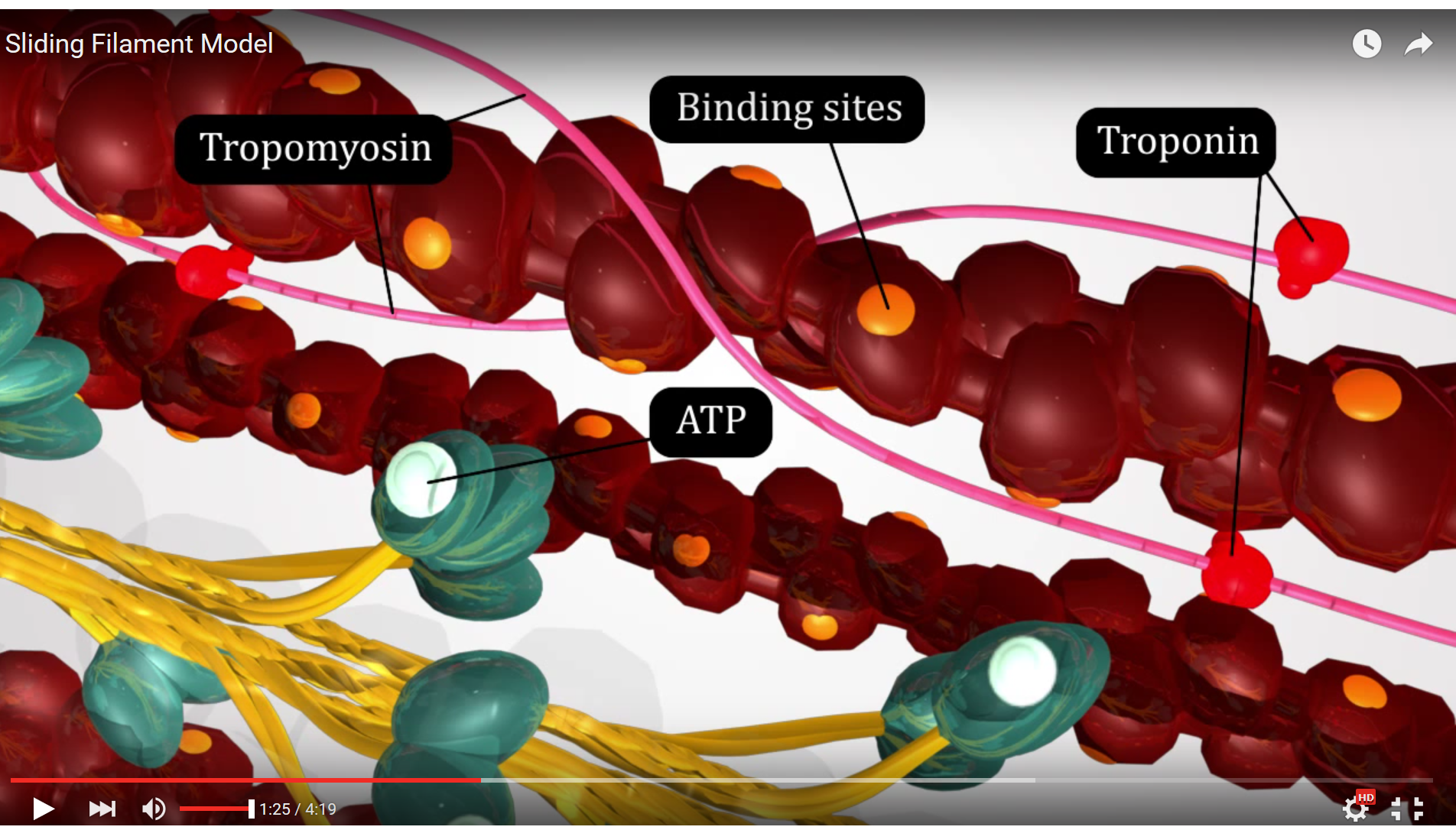
Figure 3. This screenshot from the Sliding Filament Model animation shows the landmarks described in the paragraph above.
The first step in the Sliding Filament Theory is hydrolysis of ATP on the myosin head by the enzyme ATPase, a portion of the myosin protein that has enzymatic function. The ATP attached to the myosin head is broken down by this enzyme in a hydrolysis reaction, a process utilizing adding water to the ATP breaking the high energy phosphate bond between phosphate groups and forming an ADP and an inorganic phosphate. The energy released by the hydrolysis reaction is harnessed by the myosin filament to distort the hinge region and place the myosin head in alignment with the binding site on the actin filament - this is known as the "ready" or "cocked" position.
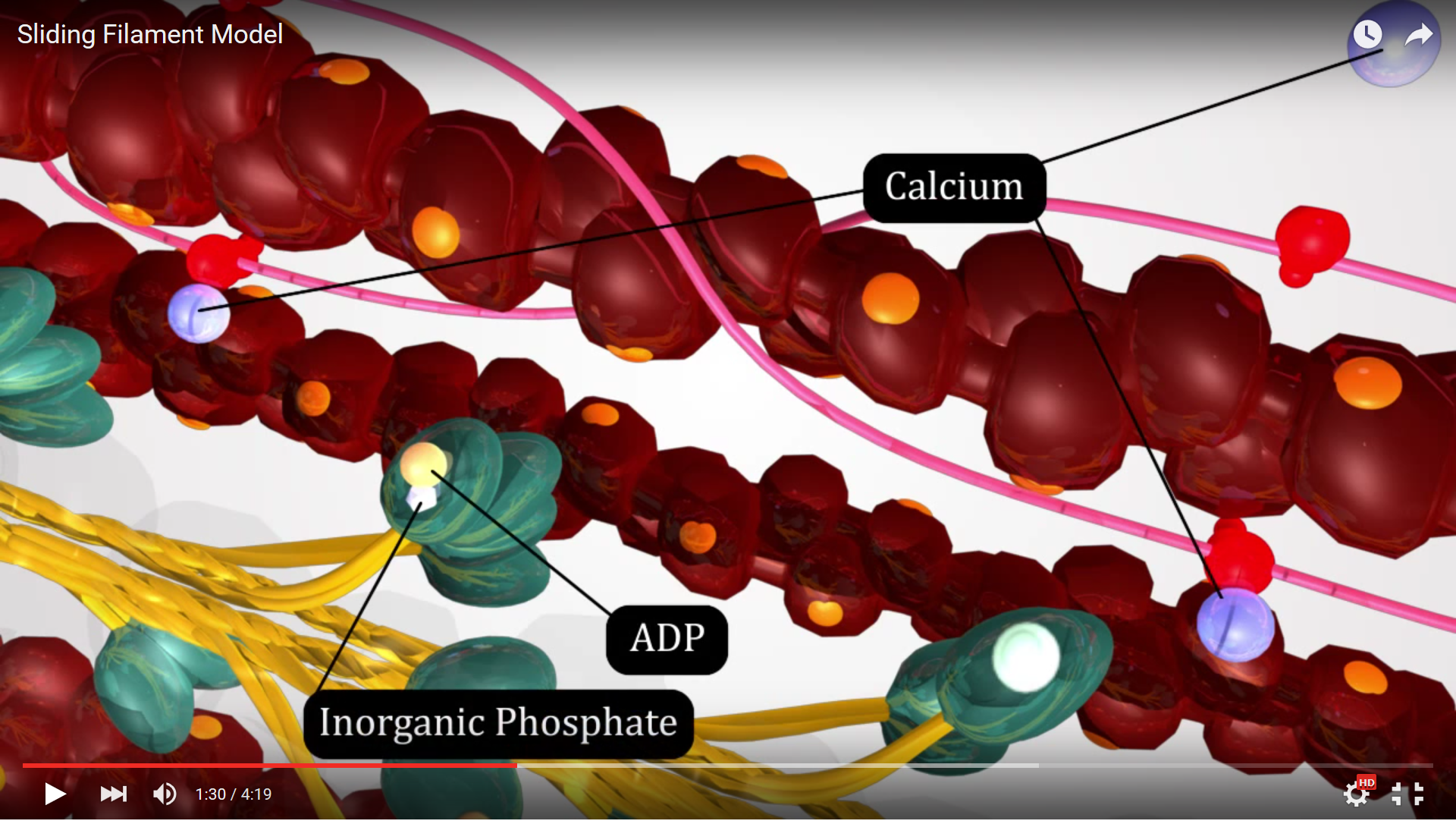
Figure 4. This screenshot from the Sliding Filament Model animation shows the landmarks described in the paragraph above.
Now that they are aligned in the ready position, the second step of Sliding Filament Theory can occur which is attachment of the myosin heads to the binding sites on actin. This attachment is commonly known as a cross bridge since the two filaments are joined at this location. The cross bridge produces tension but does not shorten the sarcomere which is our ultimate goal.
To shorten the sarcomere we move on to step 3 of Sliding Filament Theory which is the power stroke. During this step the myosin head releases the inorganic phosphate which changes the conformation of the myosin protein moving the hinge region and causing movement. The power stroke motion is caused when the myosin head changes conformation and moves it pulls the actin toward the M line since as we saw in Sliding Filament Theory step 2 they are connected. Think of this like a spring (representing myosin) and at the end of the spring is where actin would be attached. Step 2 is compressing the spring and step 3 is releasing the spring. This mechanical energy created moves the actin and makes the sarcomere shorter. However, each sarcomere needs to shorten from a varying starting length (varies due to cell type and muscle being studied) to approximately 2.7 um. Each power stroke only shortens the sarcomere 10nm. So many sequential contractions are needed to reach full contraction and produce enough tension to do work. This means that after step 3 has concluded, the myosin head will have to detach from the actin to start the Sliding Filament Theory all over again.

Figure 5. This screenshot from the Sliding Filament Model animation shows the landmarks described in the paragraph above.
Step 4 of the Sliding Filament Theory is detachment of the myosin head from the actin filament. This occurs because the myosin is presented with an ATP molecule from the sarcoplasm and this molecule binds to the active site on the myosin head. The binding of ATP alters myosin's affinity for actin and the two filaments detach from one another. Once they have detached the Sliding Filament Theory can begin all over again...
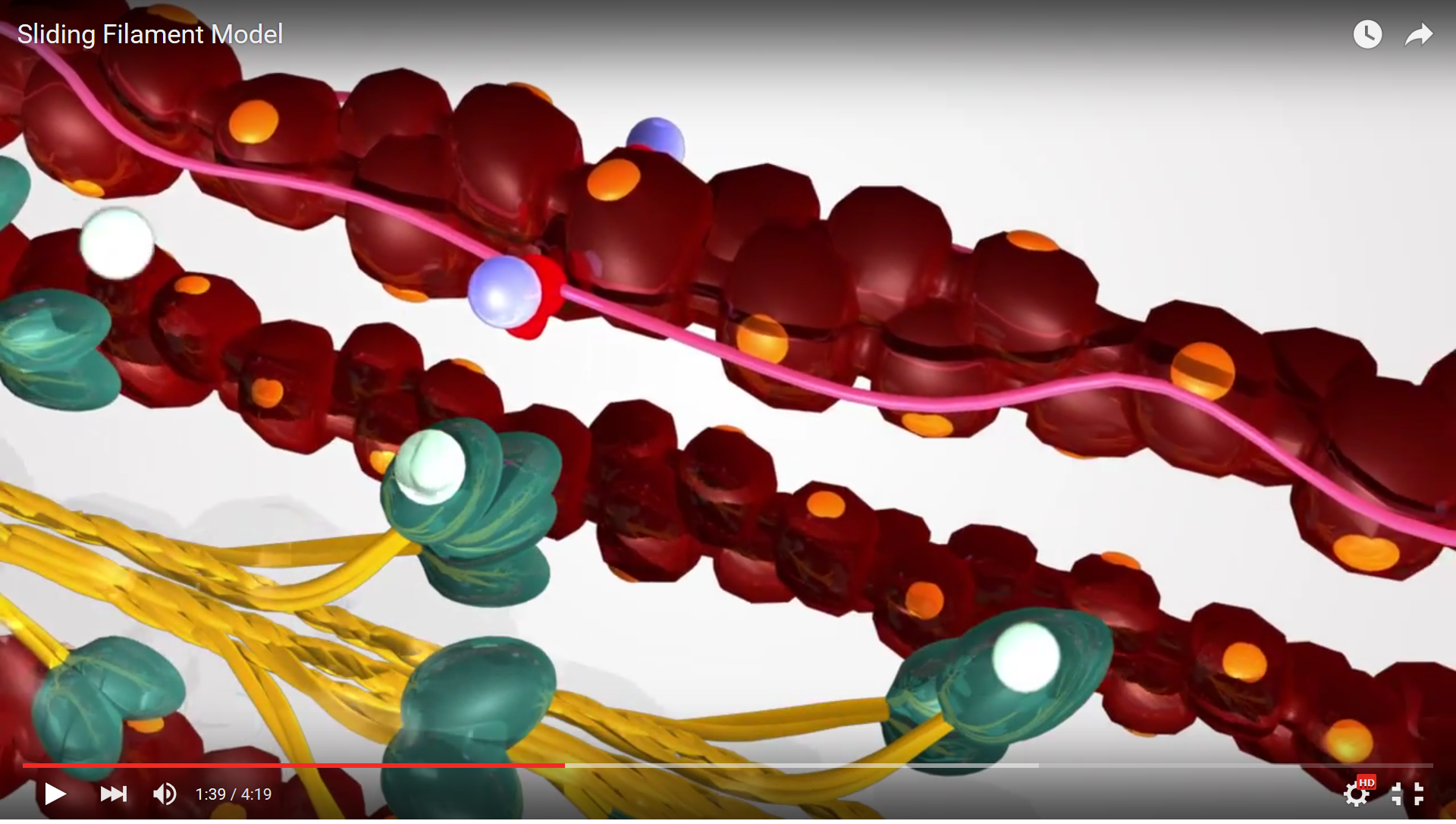
Figure 6. This screenshot from the Sliding Filament Model animation shows the landmarks described in the paragraph above.
Now view the full animation again, so you can see these steps in action.
![]()
![]()
Animation 7. View the YouTube animation Sliding Filament Model of Muscle Contration (opens in new window)
In summary, the steps of the SFT are:
- Hydrolysis of ATP to place myosin heads in the "ready" position.
- Attachment of myosin to actin at the now available binding sites - these sites remain open as long as calcium remains present.
- Power stroke causing sarcomere shortening.
- Detachment of myosin from actin when the thick filament binds to new available ATP molecules.

