Enzymes
Enzymes catalyze chemical reactions by acting upon another molecule, the reactant or "substrate(s)". In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or more products, which are then released from the active site. The active site is now free to accept another substrate molecule. In the case of more than one substrate, these may bind in a particular order to the active site, before reacting together to produce products.
The general equation representing an enzyme-substrate reaction is:
Substrate + Enzyme → Substrate — Enzyme Complex → Enzyme — Product Complex → Product + Enzyme
It is important to note that the enzyme is unchanged by the reaction. By increasing the substrate concentration, the rate of reaction will increase due to the likelihood of increase in the number of enzyme-substrate complexes; this occurs until the enzyme concentration becomes the limiting factor.
Catalysts cannot make a reaction happen that would not happen on its own. However, they can make reactions happen under less extreme conditions, or help reactions occur that would take extremely long time without a catalyst. Cells must perform and control many reactions, most of which require catalysts of some kind. For most of these reactions, cells use specialized proteins called enzymes as the catalyst. (For now the details of how enzymes catalyze reactions are not important; just understand that enzymes are cellular catalysts.)
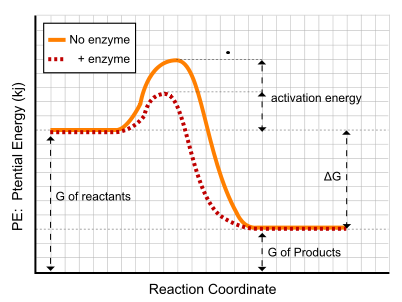
Figure 2. In this diagram, you are shown the energy needed for a reaction to occur with no enzyme (the orange line) verses the energy needed for the reaction when an enzyme is used as a catalyst (the red dashed line). Note that the activation energy is lower when an enzyme is used as a catalyst.
Within cells, the enzymes function is to accelerate various biochemical reactions. For example, sucrose hydrolysis is sped up by an enzyme.
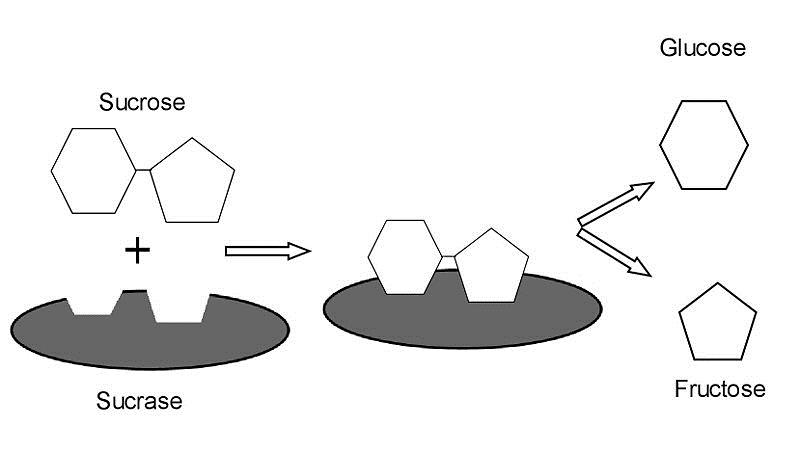
Figure 3. In this example, the hydrolysis of sucrose, C12H22O11, is catalyzed by the enzyme sucrase (the grey oval). The enzyme acts only to accelerate the breakdown of sucrose into glucose, C6H12O6, and fructose, C6H12O6.
CC BY SA: Clarie Hollenbeck, PhD and the Department of Nutriction and Food Science at San Jose State University.
Although spontaneous reactions occur without the input of energy, the rate of the reaction may be very slow. Enzymes speed up the rate of a reaction by acting as a catalyst. Most enzymes are proteins, but some RNA molecules can also perform catalytic functions. Each enzyme is characterized by its high specificity to a particular substrate. Some enzymes can couple thermodynamically favorable and unfavorable reactions.
Virtually all enzymes are proteins, but not all proteins are enzymes and other molecules such as RNA can also catalyze reactions. The most remarkable characteristics of enzymes are their ability to accelerate chemical reactions and their specificity for a particular substrate. Enzymes take advantage of the full range of intermolecular forces, (van der Waals interactions, polar interactions, hydrophobic interactions, and hydrogen bonding), to bring substrates together in most optimal orientation so that reaction will occur.
To work correctly, enzyme molecules must have a very specific shape. That shape is determined by the amino acid sequence in the protein, and the shape forms when the proteins folds up after translation. As an enzyme folds, it forms an active site where chemical substrate(s) fit.

