Enzyme Inhibition
Most enzymes have an active site that is substrate specific, meaning the active site can bind and catalyze a reaction for one or a few different substrates. Substrate specificity depends on the shape of the active site where the substrate binds. If the shape of the active site changes slightly, the substrate may not bind anymore. And even if the substrate is able to bind, an enzyme with an altered shape may not be able to carry out its normal function of catalyzing a specific chemical reaction.
Enzyme inhibitors are small molecules that bind directly to enzyme molecules and change the shape of the enzyme in some way, which slows or stops the enzyme from converting substrates into products. Inhibitors work in several different ways.
- Irreversible inhibitors bind to enzyme molecules in a simple chemical reaction that permanently blocks the enzyme's functioning. Cells or entire organisms must therefore produce more of the enzyme before they can process substrate again. For example, nerve gas functions as an irreversible inhibitor, permanently blocking pathways involved in nerve message transmission, which results in the death of any exposed organism.
- Reversible inhibitors bind temporarily to enzyme molecules, and so block enzyme function only temporarily. Where they bind determines how they affect enzyme function.
Example: Inhibitors
Competitive inhibitors are molecules that fit in the enzyme binding site just like the normal substrate. If an enzyme is added to a mixture of the normal substrate and the inhibitor, some enzyme molecules will bind the substrate and others the inhibitor. Since the two molecules are competing for the enzyme molecules, whichever molecule is in the highest concentration usually binds most often.
Competitive inhibitor molecules do not undergo a chemical reaction, (bind and change the shape of the enzyme), only normal substrate molecules do. The net result of competitive inhibition is that less product is made.
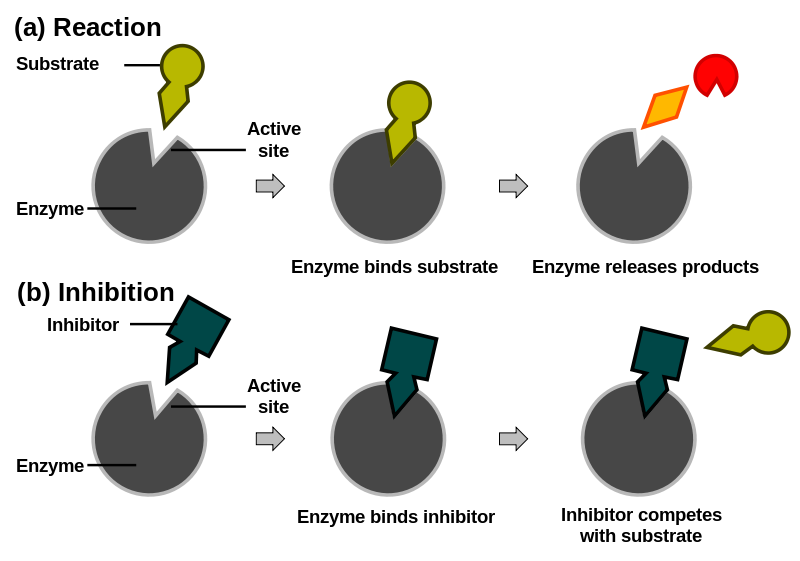
Figure 6. In this generalized diagram the top example shows how a substrate (olive green) typically binds to an enzyme (grey) at the active site. The reaction proceeds and the products (orange) are released. In the bottom panel, a competitor molecule (dark green) is in the active site normally occupied by the natural substrate therefore inhibiting the reaction.
Many important competitive inhibitors of enzymes have been discovered. For example, sulfonamide was one of the first commercial drugs for treating bacterial infections. Bacteria use the enzyme dihydropteroate synthetase, (DHPS), to convert 4-aminobenzoic acid to folic acid, an essential coenzyme. Sulfonamide binds to the same active site on DHPS as the normal substrate, and competitively inhibits the enzyme. As a result of this process of inhibition, the bacteria are "starved" of folate and die. Non-competitive inhibitors are another type of reversible inhibitors. They are molecules that bind to enzyme molecules somewhere other than the main substrate binding site. If enzyme is added to a mixture of the normal substrate and non-competitive inhibitor, all of the binding sites on the enzyme molecules will be available to bind the substrate. However, the inhibitor molecules attach to another site and slow down enzyme activity. Once again, fewer products are made.
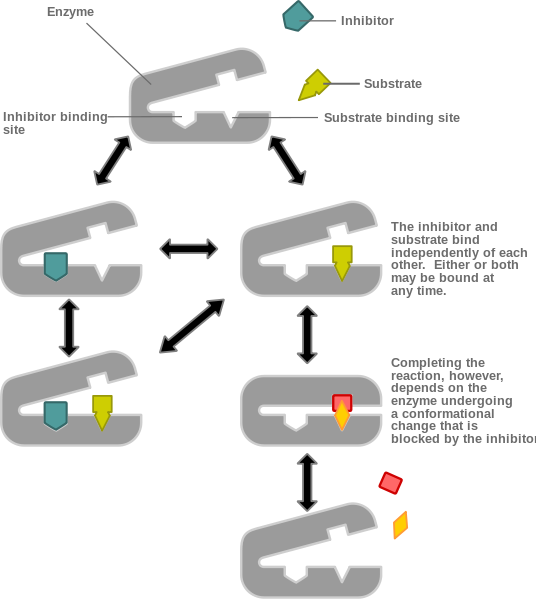
Figure 7. A non-competitive inhibitor. The above diagram shows non-competitive inhibition. Here inhibitor molecules (teal) bind to sites other than the active binding site of the main substrate (yellow). The inhibitor (teal) and substrate (yellow) bind independently and either or both may be bound at any time. When an inhibitor molecule (teal) binds to the enzyme (grey), such as in the left pathway, it prevents the enzyme (grey) from undergoing the conformational change needed to complete the reaction with the substrate (yellow). The non-inhibited reaction is shown on the right where the enzyme (grey) binds with the substrate (yellow) and completes the reaction then releasing two products, shown here as an orange diamond and a red square.
This video discusses enzymes from a macromolecule and metabolic perspective:
![]()
![]()
Video 1. Enzymes: A Macromolecule Perspective video (opens Youtube in new window)


