Covalent Bonds
Covalent bonds form when two atoms share pairs of electrons to achieve the octet rule. The shared electrons orbit the nuclei of both the bonding atoms; the overlap of electron clouds is the bond. Covalently bonded atoms form discrete particles known as molecules. A molecule is a group of two or more atoms held together by covalent bonding. A molecule will always have a set number of atoms. For example, in a water molecule, one atom of oxygen forms 2 covalent bonds with 2 Hydrogen atoms. Thus each water molecule (H2O) has exactly 1 atom of Oxygen and 2 atoms of Hydrogen.
In some cases it is necessary for two atoms to share more than just one pair of electrons between them to achieve an octet. If one pair of electrons (2 electrons) is shared between two atoms it is called a single bond. If two pairs of electrons (4 electrons) are shared between two atoms, it is called a double bond. If three pairs of electrons (6 electrons) are shared between two atoms, it is called a triple bond.
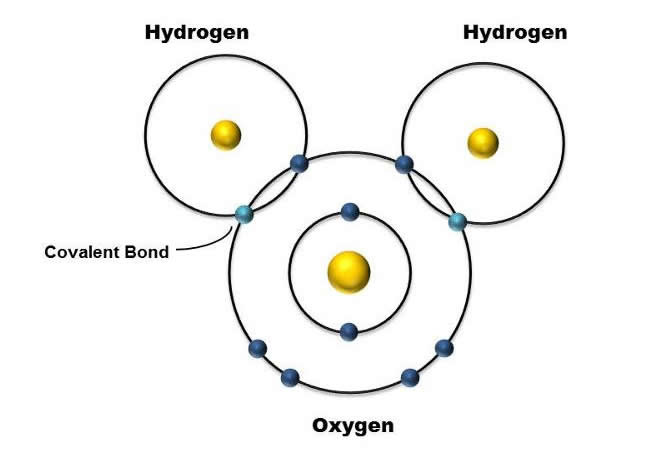
Figure 1.Covalent Bonds of H2O. The protons, represented by a yellow ball, are surrounded by blue electron balls. The two lighter blue shaded electrons represent the two electrons shared between oxygen and hydrogen.
Polar Covalent Bonds
There are two types of covalent bonds: polar and nonpolar. In a polar covalent bond, the electrons are unequally shared by the atoms because they are more attracted to one nucleus than the other. The relative attraction of an atom to an electron is known as its electronegativity: atoms that are more attracted to an electron are considered to be more electronegative Because of the unequal distribution of electrons between the atoms of different elements, a slightly positive (δ+) or slightly negative (δ–) charge develops. This partial charge is known as a dipole; this is an important property of water and accounts for many of its characteristics. The dipole in water occurs because oxygen has a higher electronegativity than hydrogen, which means that the shared electrons spend more time in the vicinity of the oxygen nucleus than they do near the nucleus of the hydrogen atoms.
Nonpolar Covalent Bonds
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share electrons equally. For example, molecular oxygen (O2) is nonpolar because the electrons will be equally distributed between the two oxygen atoms. The four bonds of methane are also considered to be nonpolar because the electronegativities of carbon and hydrogen are nearly identical.
