Concentrations of Solutes
Various mixtures of solutes and water are described in chemistry. The concentration of a given solute is the number of particles of that solute in a given space (oxygen makes up about 21 percent of atmospheric air). In the bloodstream of humans, glucose concentration is usually measured in milligram (mg) per deciliter (dL), and in a healthy adult averages about 100 mg/dL. Another method of measuring the concentration of a solute is by its molarilty—which is moles (M) of the molecules per liter (L). The mole of an element is its atomic weight, while a mole of a compound is the sum of the atomic weights of its components, called the molecular weight. An often-used example is calculating a mole of glucose, with the chemical formula C6H12O6. Using the periodic table, the atomic weight of carbon (C) is 12.011 grams (g), and there are six carbons in glucose, for a total atomic weight of 72.066 g. Doing the same calculations for hydrogen (H) and oxygen (O), the molecular weight equals 180.156g (the "gram molecular weight" of glucose). When water is added to make one liter of solution, you have one mole (1M) of glucose. This is particularly useful in chemistry because of the relationship of moles to "Avogadro's number." A mole of any solution has the same number of particles in it: 6.02 × 1023. Many substances in the bloodstream and other tissue of the body are measured in thousandths of a mole, or millimoles (mM). A colloid is a mixture that is somewhat like a heavy solution. The solute particles consist of tiny clumps of molecules large enough to make the liquid mixture opaque (because the particles are large enough to scatter light). Familiar examples of colloids are milk and cream. In the thyroid glands, the thyroid hormone is stored as a thick protein mixture also called a colloid. A suspension is a liquid mixture in which a heavier substance is suspended temporarily in a liquid, but over time, settles out. This separation of particles from a suspension is called sedimentation. An example of sedimentation occurs in the blood test that establishes sedimentation rate, or sed rate. The test measures how quickly red blood cells in a test tube settle out of the watery portion of blood (known as plasma) over a set period of time. Rapid sedimentation of blood cells does not normally happen in the healthy body, but aspects of certain diseases can cause blood cells to clump together, and these heavy clumps of blood cells settle to the bottom of the test tube more quickly than do normal blood cells.
The Role of Water in Chemical Reactions
Two types of chemical reactions involve the creation or the consumption of water:
- Dehydration synthesis
- Hydrolysis.
In dehydration synthesis, one reactant gives up an atom of hydrogen and another reactant gives up a hydroxyl group (OH) in the synthesis of a new product. In the formation of their covalent bond, a molecule of water is released as a byproduct (Figure 4). This is also sometimes referred to as a condensation reaction.
In hydrolysis, a molecule of water disrupts a compound, breaking its bonds. The water is itself split into H and OH. One portion of the severed compound then bonds with the hydrogen atom, and the other portion bonds with the hydroxyl group. These reactions are reversible, and play an important role in the chemistry of organic compounds (which will be discussed shortly).
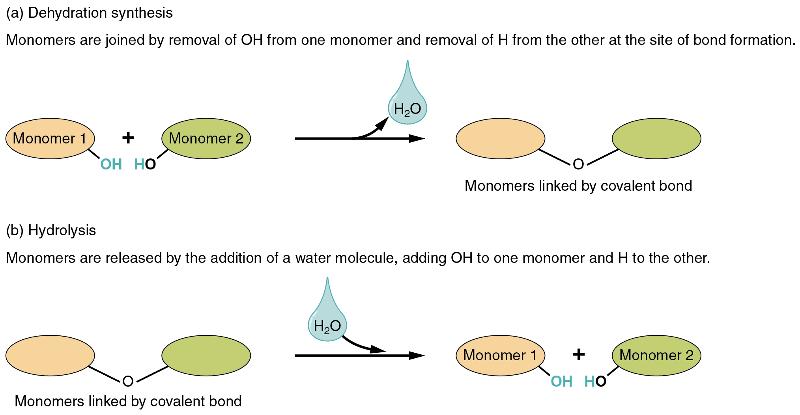
Figure 4 Dehydration Synthesis and Hydrolysis Monomers, the basic units for building larger molecules, form polymers (two or more chemically-bonded monomers). (a) In dehydration synthesis, two monomers are covalently bonded in a reaction in which one gives up a hydroxyl group and the other a hydrogen atom. A molecule of water is released as a byproduct during dehydration reactions. (b) In hydrolysis, the covalent bond between two monomers is split by the addition of a hydrogen atom to one and a hydroxyl group to the other, which requires the contribution of one molecule of water.
CC BY: Openstax College
Salts
Recall that salts are formed when ions form ionic bonds. In these reactions, one atom gives up one or more electrons, and thus becomes positively charged, whereas the other accepts one or more electrons and becomes negatively charged. You can now define a salt as a substance that, when dissolved in water, dissociates into ions other than H+ or OH– . This fact is important in distinguishing salts from acids and bases, discussed next. A typical salt, NaCl, dissociates completely in water (Figure 5). The positive and negative regions on the water molecule (the hydrogen and oxygen ends respectively) attract the negative chloride and positive sodium ions, pulling them away from each other. Again, whereas nonpolar and polar covalently bonded compounds break apart into molecules in solution, salts dissociate into ions. These ions are electrolytes; they are capable of conducting an electrical current in solution. This property is critical to the function of ions in transmitting nerve impulses and prompting muscle contraction.
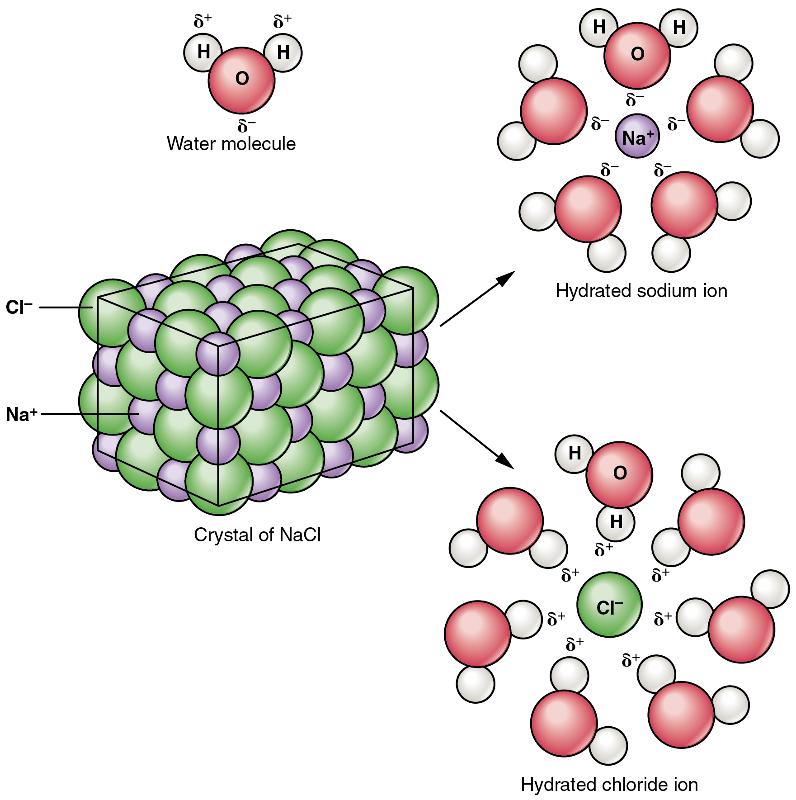
Figure 5 Dissociation of Sodium Chloride in Water: Notice that the crystals of sodium chloride dissociate not into molecules of NaCl, but into Na+ cations and Cl– anions, each completely surrounded by water molecules.
CC BY: Openstax College
Many other salts are important in the body. For example, bile salts produced by the liver help break apart dietary fats, and calcium phosphate salts form the mineral portion of teeth and bones.