Hydrogen Bonds and Van der Waals Interactions
Not all bonds are ionic or covalent; weaker bonds can also form between molecules. Two types of weak bonds that frequently occur are hydrogen bonds and van der Waals interactions. Without these two types of bonds, life as we know it would not exist.
Hydrogen bonds occur when there is an attraction between two polar molecules, a hydrogen atom and an electronegative atom (e.g. nitrogen, oxygen). Polar molecules, in this instance, are molecules in which the electrons are not shared equally among covalently bonded atoms, resulting in part of the molecule being slightly positive and the other being slightly negative. The figure below illustrates the interaction of hydrogen bonding in water.
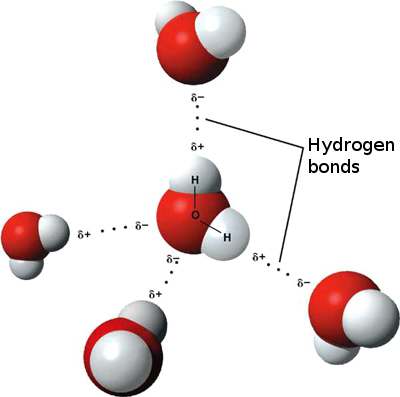
Figure 2.: Hydrogen bonds between water molecules. The two white hydrogen atoms are covalently bonded to the red oxygen atom. Dotted lines show the hydrogen bonds between the white hydrogens and the red oxygen of a neighboring molecule.
CC BY SA: Michael Manas
Like hydrogen bonds, Van der Waals interactions are weak interactions between molecules. Van der Waals attractions can occur between any two or more molecules and are dependent on slight fluctuations of the electron densities, which can lead to slight temporary dipoles around a molecule. For these attractions to happen, the molecules need to be very close to one another. These interactions are said to be responsible for geckos ability to cling to and climb glass as seen in the figure below.
Activity not available on mobile devices (description)
